
|
个人简介Personal Profile
本科和博士毕业于华中师范大学化学学院,获华中师范大学优秀博士论文;2011年-2014年在美国马里兰大学从事博士后研究;2014年加入西北大学化学与材料科学学院,教授/博导、副院长。先后荣获陕西省青年百人计划、霍英东青年教师基金、陕西省杰出青年科学基金、国家自然科学基金优秀青年科学基金。主要围绕基于大环分子的超分子化学领域开展研究工作,发展了在生物兼容环境中仿生超分子体系的构建及针对生物分子的精准识别与医学诊疗应用;迄今以第一或通讯作者发表J. Am. Chem. Soc., Angew. Chem. Int. Ed., CCS Chem.等期刊论文六十余篇;相关研究成果获陕西高等学校科学技术研究优秀成果一等奖(排名第一)和陕西省自然科学一等奖(排名第三)。
学习和工作经历:
2014年-今:教授/博导,西北大学,化学与材料科学学院
2017年-2018年:国家公派访问学者,美国犹他大学,化学系,合作导师:Peter Stang 院士
2011年-2014年:博士后,美国马里兰大学,化学与生物化学系,合作导师:Lyle Isaacs 教授
2006年-2011年:理学博士(有机化学),华中师范大学,化学学院,导师:吴安心 教授
2002年-2006年:理学学士(应用化学),华中师范大学,化学学院
基金项目:
2021年国家自然科学基金优秀青年科学基金
2020年陕西省杰出青年科学基金
2020年霍英东高校青年教师基金
2016年陕西省青年百人计划
荣誉奖项:
2025年“全球前2%顶尖化学科学家”
2025年西北大学优秀博士学位论文指导教师(学生:李清芳)
2024年西北大学优秀研究生导师
2024年陕西省优秀博士学位论文指导教师(学生:程琳)
2023年陕西省优秀博士学位论文指导教师(学生:李亚雯)
2023年全国超分子化学学术讨论会学术新星奖
2023年大环芳烃超分子化学学术新星奖
2022年陕西省化学优秀青年奖
2022年西北大学科研奖
2022年陕西高等学校科学技术研究优秀成果一等奖(排名第一)
2021年陕西省自然科学一等奖(排名第三)
2021年西北大学科研奖
2021年西北大学优秀博士学位论文指导教师(学生:李亚雯)
2020年西北大学优秀硕士学位论文指导教师(学生:段红红)
2020年西北大学优秀教师
2020年Thieme Chemistry Journals Award
2011年华中师范大学优秀博士论文
学术兼职:
中国化学会第三十一届理事会超分子化学专业委员会委员
中国化学会高级会员
中国感光学会青年理事会理事
Chinese Chemical Letter高级编委
Aggregate青年顾问编委
研究兴趣:
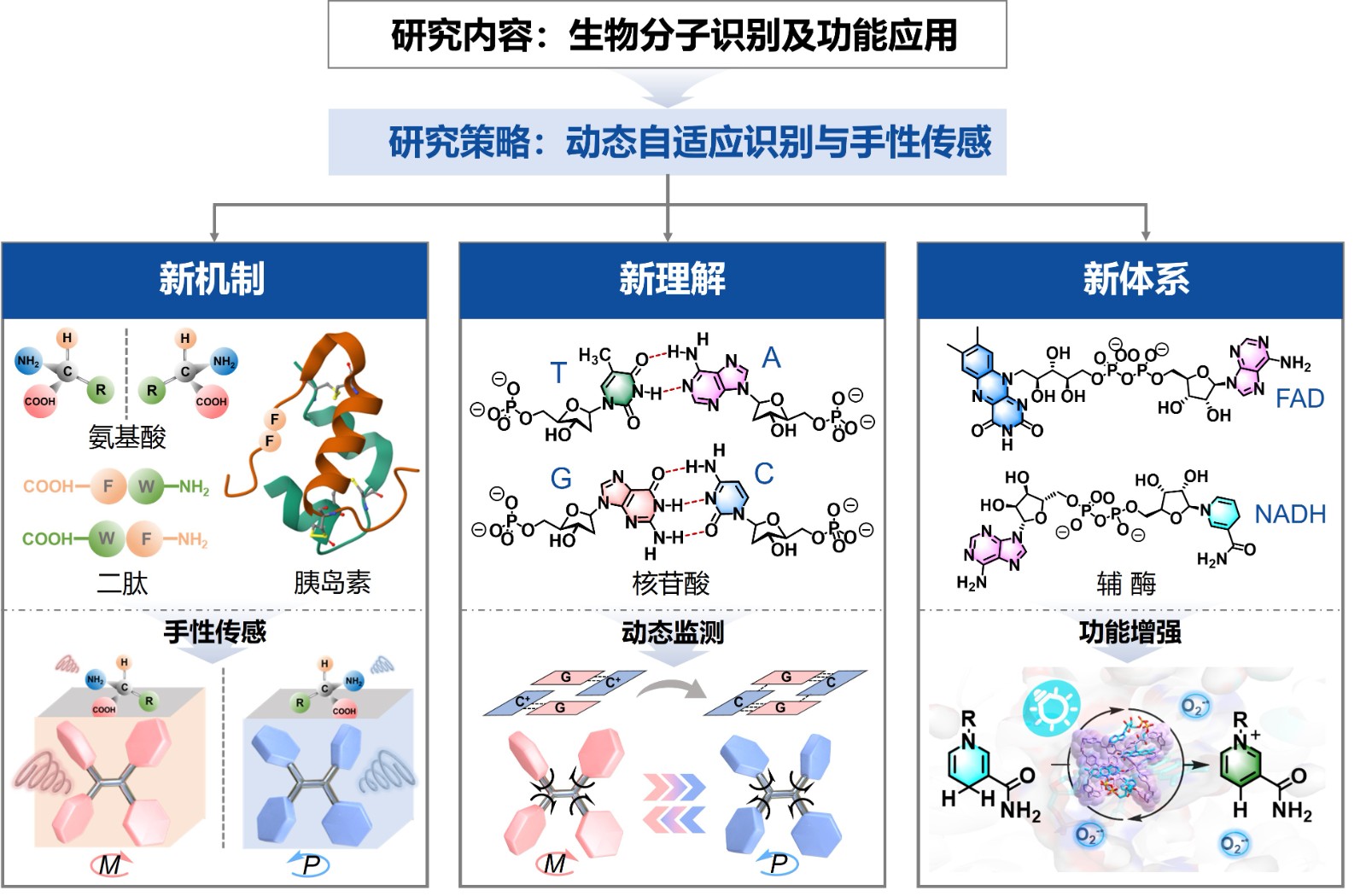
课题组针对“生物分子的水相识别行为和功能调控的分子作用机制”的科学问题,通过开发水溶性仿生大环超分子人工体系,系统开展了“生物分子识别与功能应用”的研究,提出了基于氨基酸、多肽、蛋白质的多层次手性结构的“动态自适应手性传感”的新机制,实现了针对核苷"碱基对"氢键组装及其异构化转变机理的新理解,建立了基于仿生分子识别为基础的光催化辅助光动力治疗的新体系,为生物分子检测与监测机制的基础研究及其相关医学诊疗的应用研究开辟了一条新途径。
毕业学生:
博士毕业生:
2019年:鱼洋
2021年:李亚雯、王聘聘
2022年:程琳
2023年:段红红
2025年:赵凌玉、李颖洁、李清芳、燕超超
硕士毕业生:
2017年:李杰
2018年:王聘聘
2019年:张蓓琳
2020年:李晨阳、段红红
2021年:年浩、张海洋、秦春艳
2022年:段燕娟、王玲
2023年:田萍、马焕青
2024年:宋晓雯、郭智慧、姚诺锦
2025年:王平霞、康旭豪、杨艳霞、王凯歌
本科毕业生:
2020年:敖宛彤(丹麦哥本哈根大学),金慧琳(比利时布鲁塞尔自由大学)
2024年:韩京娱(中国科学院理化技术研究所)
科研成果:
独立工作(2014-今):
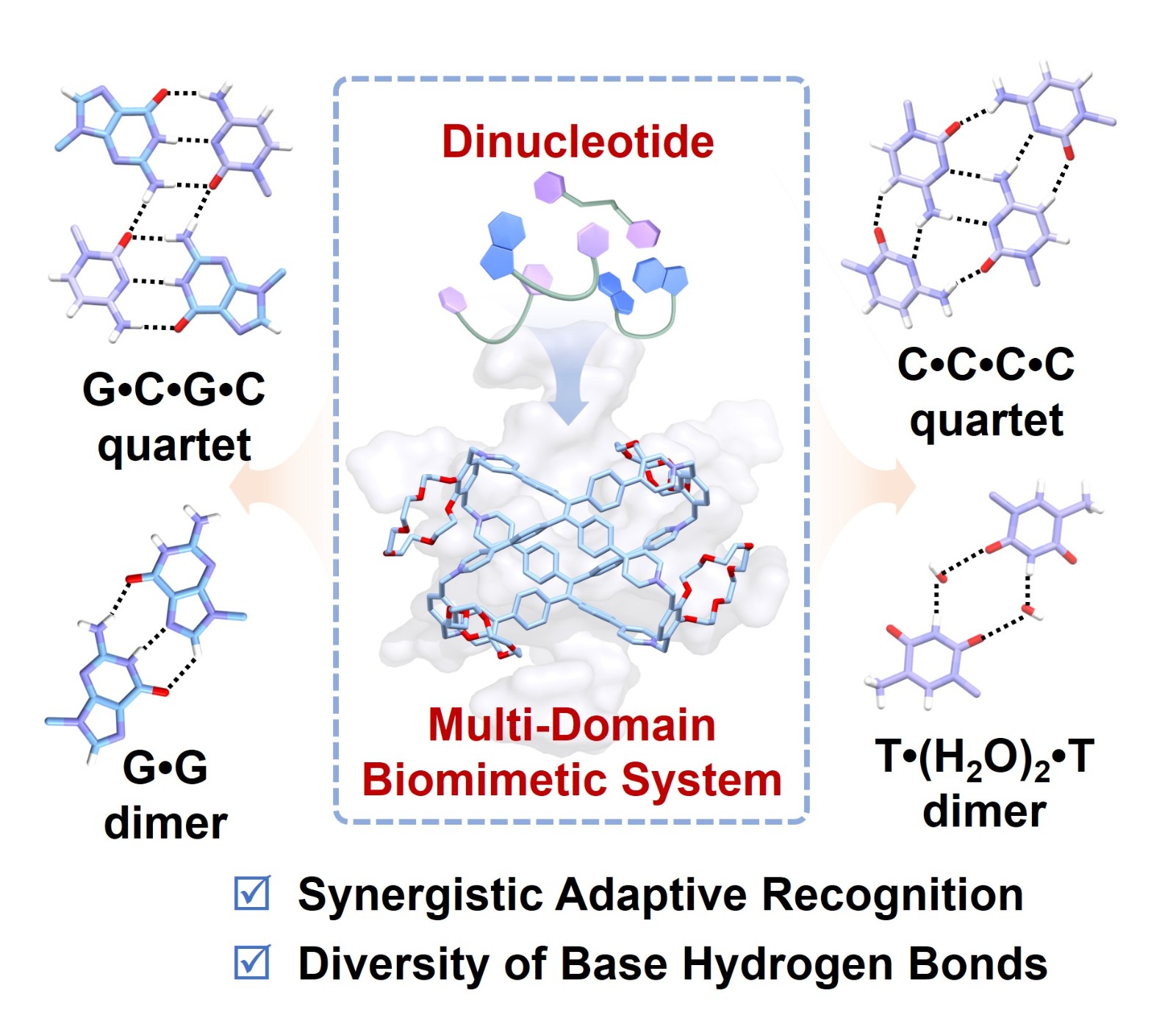
65. Cao, F.; Cao, Z.; Yao, N.; Yang, T.; He, T.; Cheng, L.*; Cao, L.*, Unraveling Biomimetic Hydrogen Bonds of Dinucleotides by a Crown-Ether-Functionalized Tetraphenylethene-Based Cage. Angew. Chem. Int. Ed. 2026, 65, e23981. (HOT PAPER)
http://doi.org/10.1002/anie.202523981

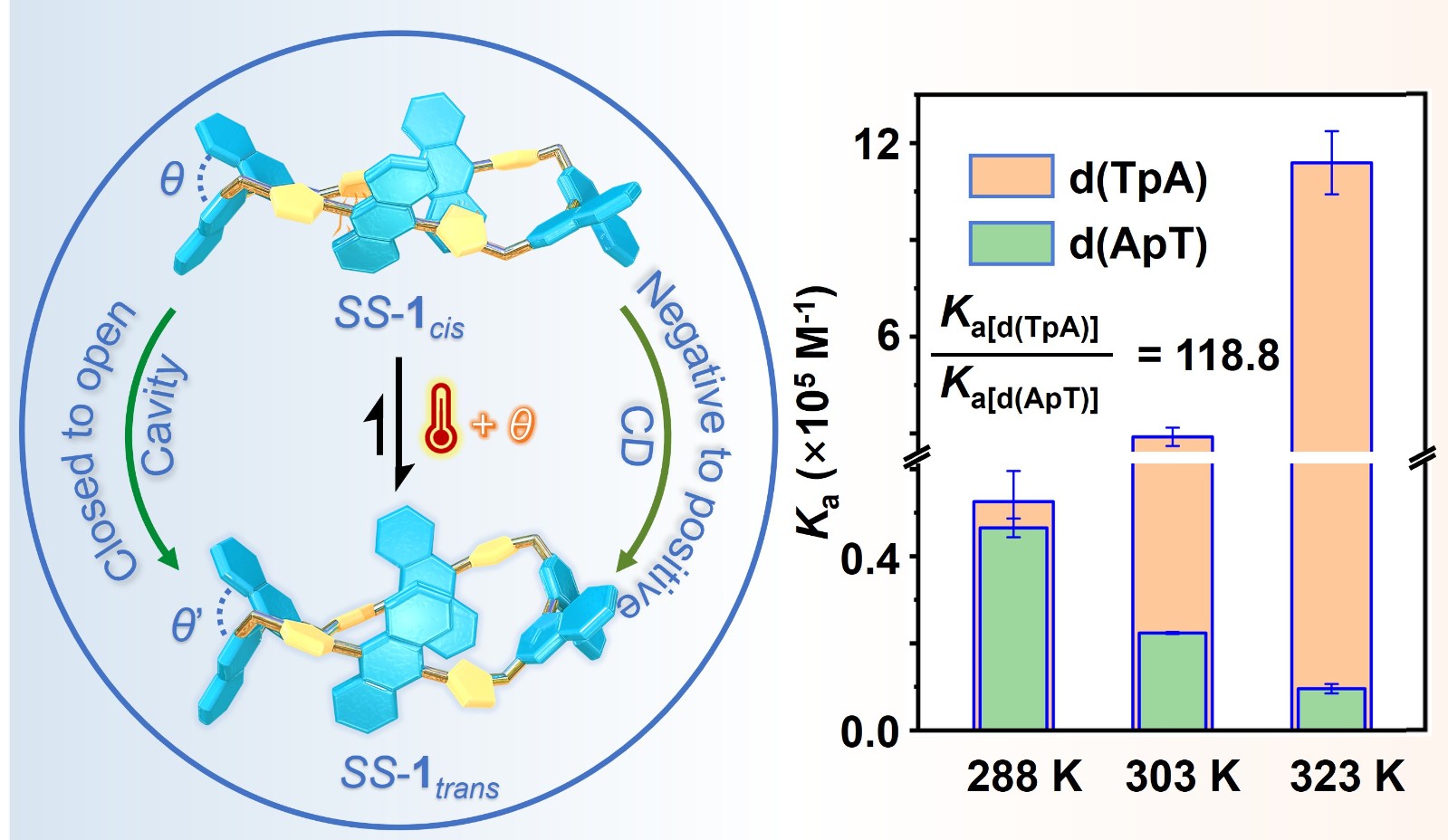
64. He, T.; Huo, M.; Wang, P.; Huo, H.; Yang, T.; Cao, F.; Zhao, L.; Yang, Y.; Cheng, L.; Cao, L.*, Temperature-Driven Conformational Switching of Binaphthalene-Based Tetraimidazolium Macrocycles to Enhance Sequence-Selective Recognition of Dinucleotides in Water. Angew. Chem. Int. Ed. 2025, 64, e202516534. (HOT PAPER)
https://onlinelibrary.wiley.com/doi/10.1002/anie.202516534
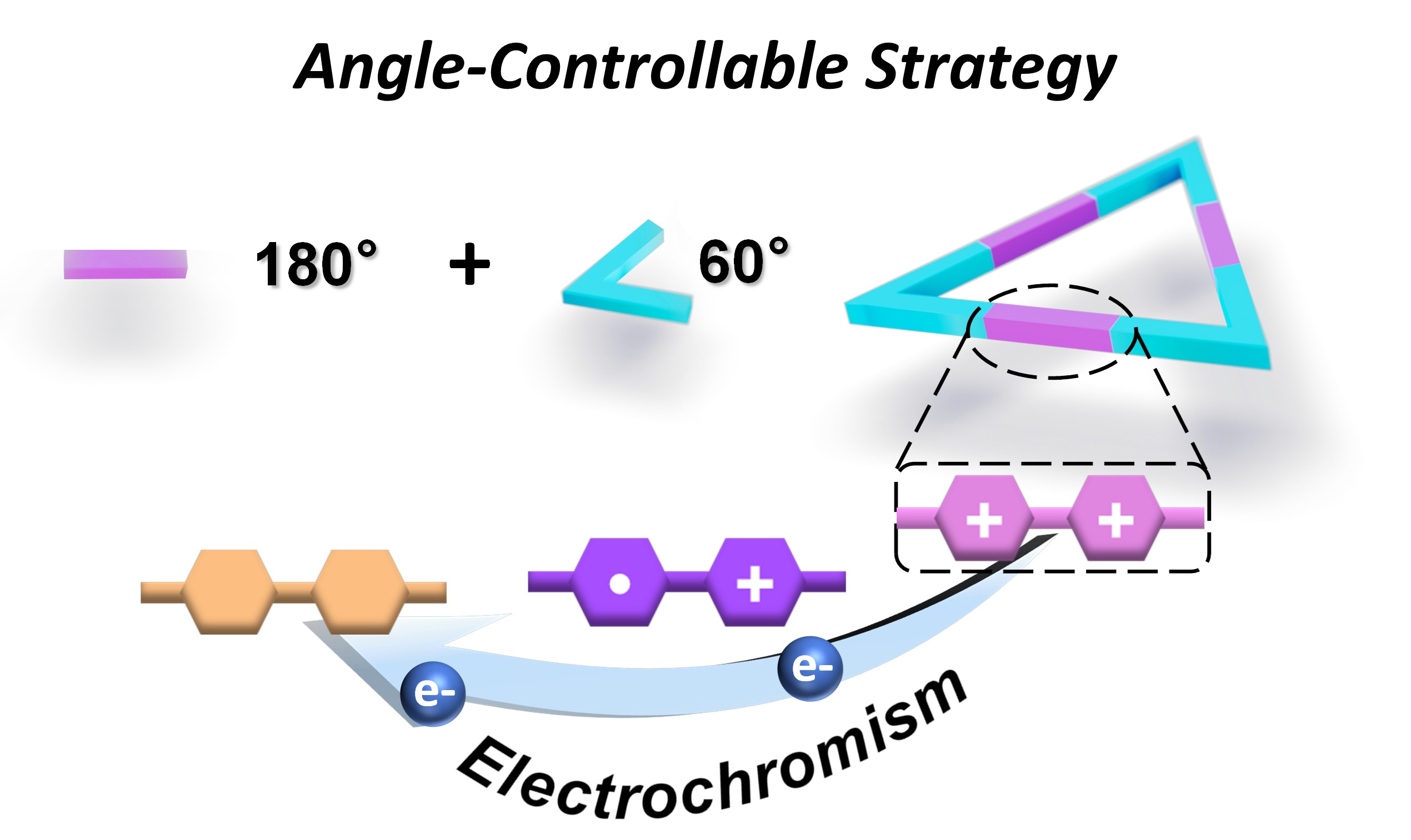
63. Yu, Y.; Song, X.; Li, Y.; Wang, P.; Cheng, L.; Yang, Y.;* He, G.;* Cao, L.*, Angle-controlled synthesis and redox property of tetraphenylethene-based hexacationic triangular macrocycle. Chem. Commun. 2025, 61, 13193-13196.
https://pubs.rsc.org/en/content/articlelanding/2025/cc/d5cc03589a
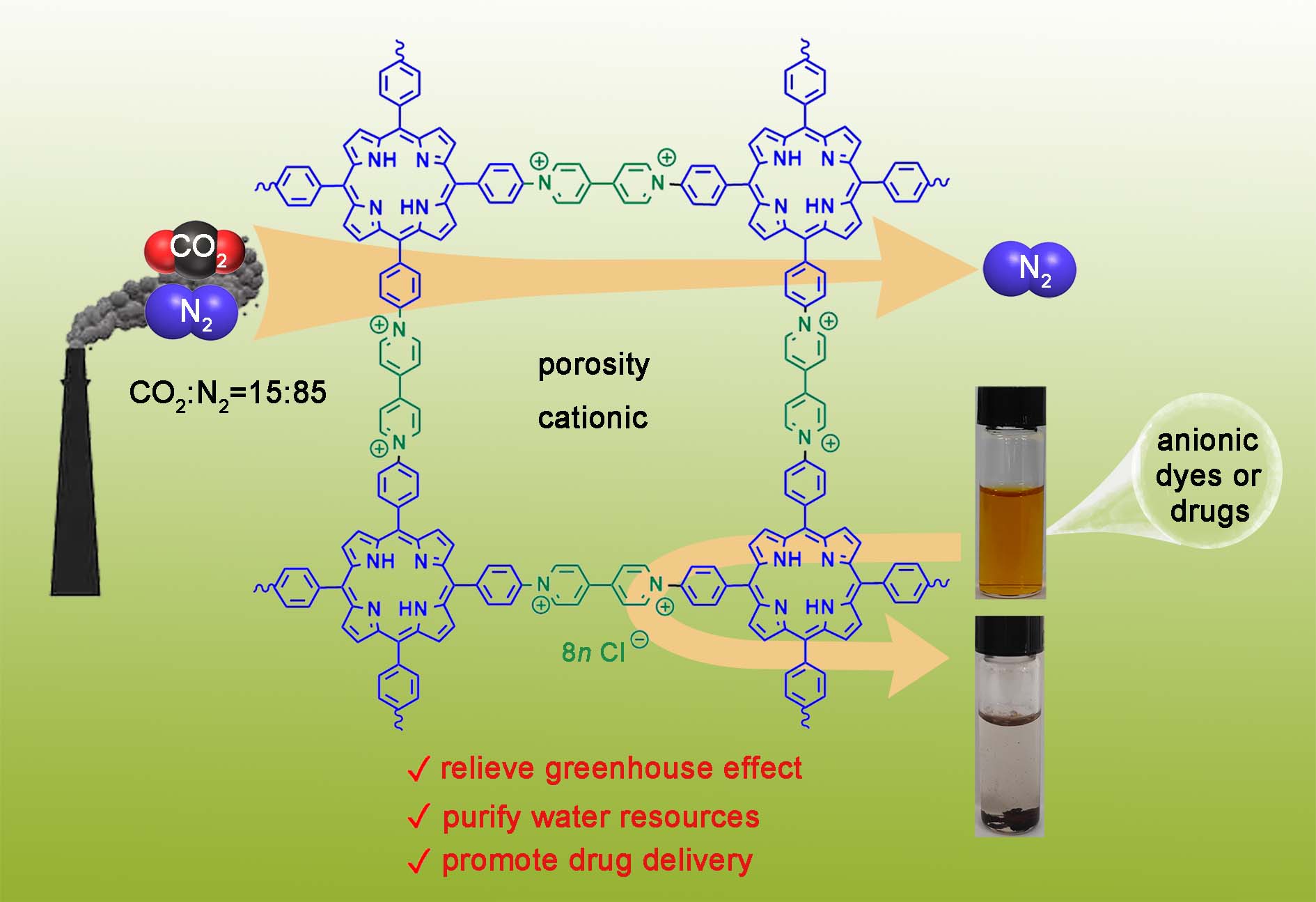
62. Wang, P.;* Zhang, K.; Chen, R.; Lu, Y.; Li, F.; Sun, T.; Song, X.; Li, X.; Cao, L.*, Cationic porous organic polymers for selective adsorption in gas and liquid phases. Polymer Chem. 2025, 16, 3129-3137.
https://pubs.rsc.org/en/content/articlelanding/2025/py/d5py00330j
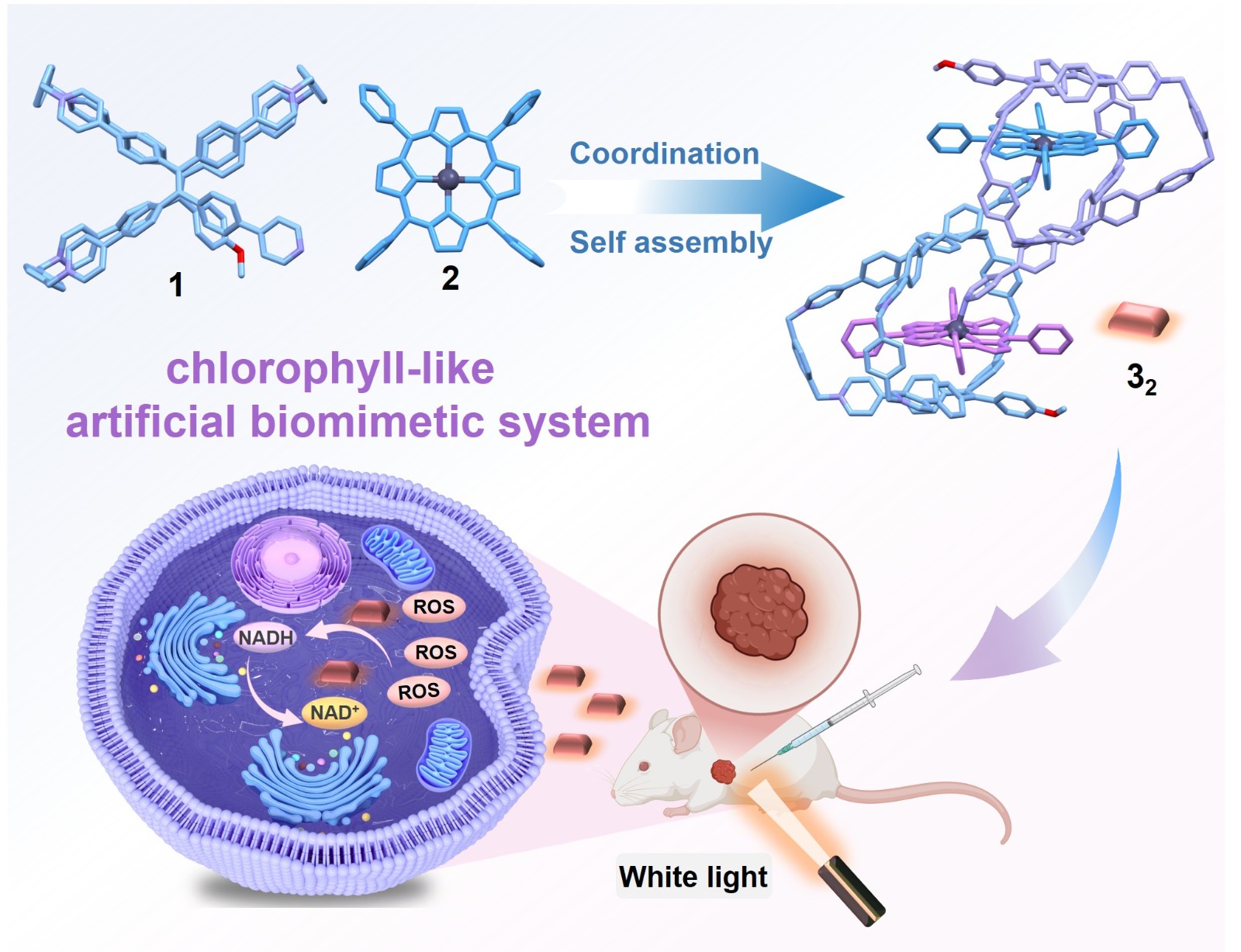
61. Cao, F.; Lin, H.; Zhang, C.; Cao, Z.; Li, Q.; Wang, P.; Gao, G.; Cheng, L.;* Tan, Y.;* Cao, L.,* Coordination-Driven [c2]Daisy Chain of Monofunctionalized Tetraphenylethene-Based Hexacationic Cage and Zinc-Porphyrin toward Photocatalysis-Assisted Photodynamic Therapy. CCS Chem. 2026, 8, 379-396.
https://www.chinesechemsoc.org/doi/10.31635/ccschem.025.202505526
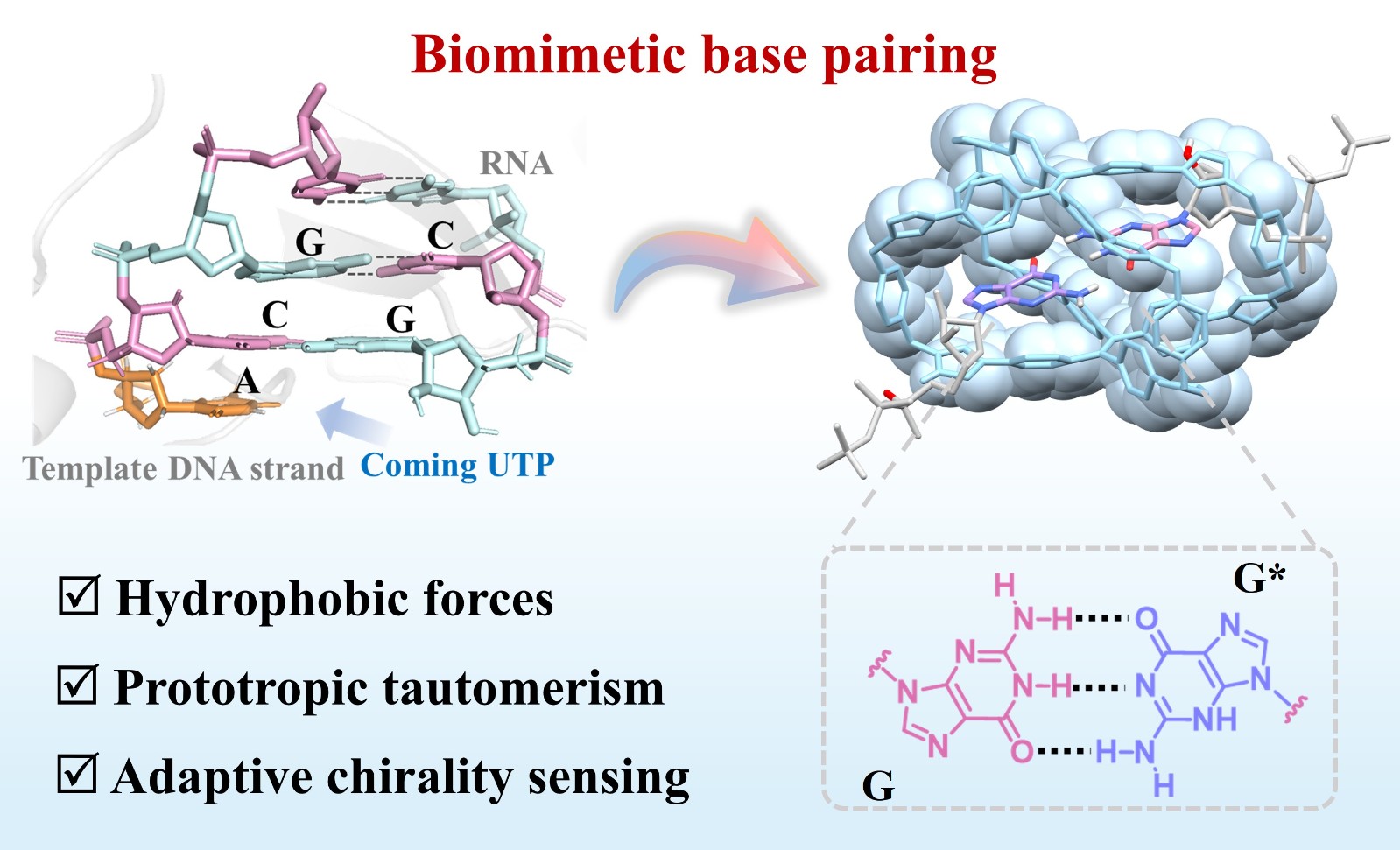
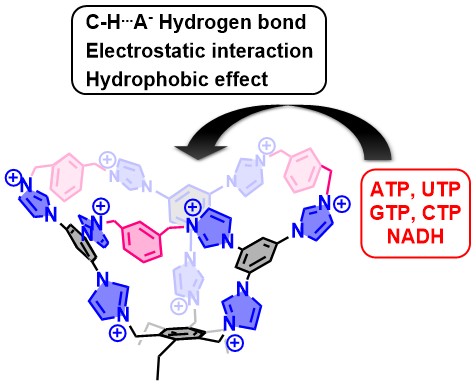
60. Li, Y.; Cheng, L.; Wang, P.; Kang, X.; Li, Q.; Cao, L.,* Biomimetic Hydrogen-Bonded Base Pairing of Nucleoside Triphosphates in Water by a Tetraphenylethene-Based Octaimidazolium Cage. Angew. Chem. Int. Ed. 2025, e202505732.
https://onlinelibrary.wiley.com/doi/10.1002/anie.202505732
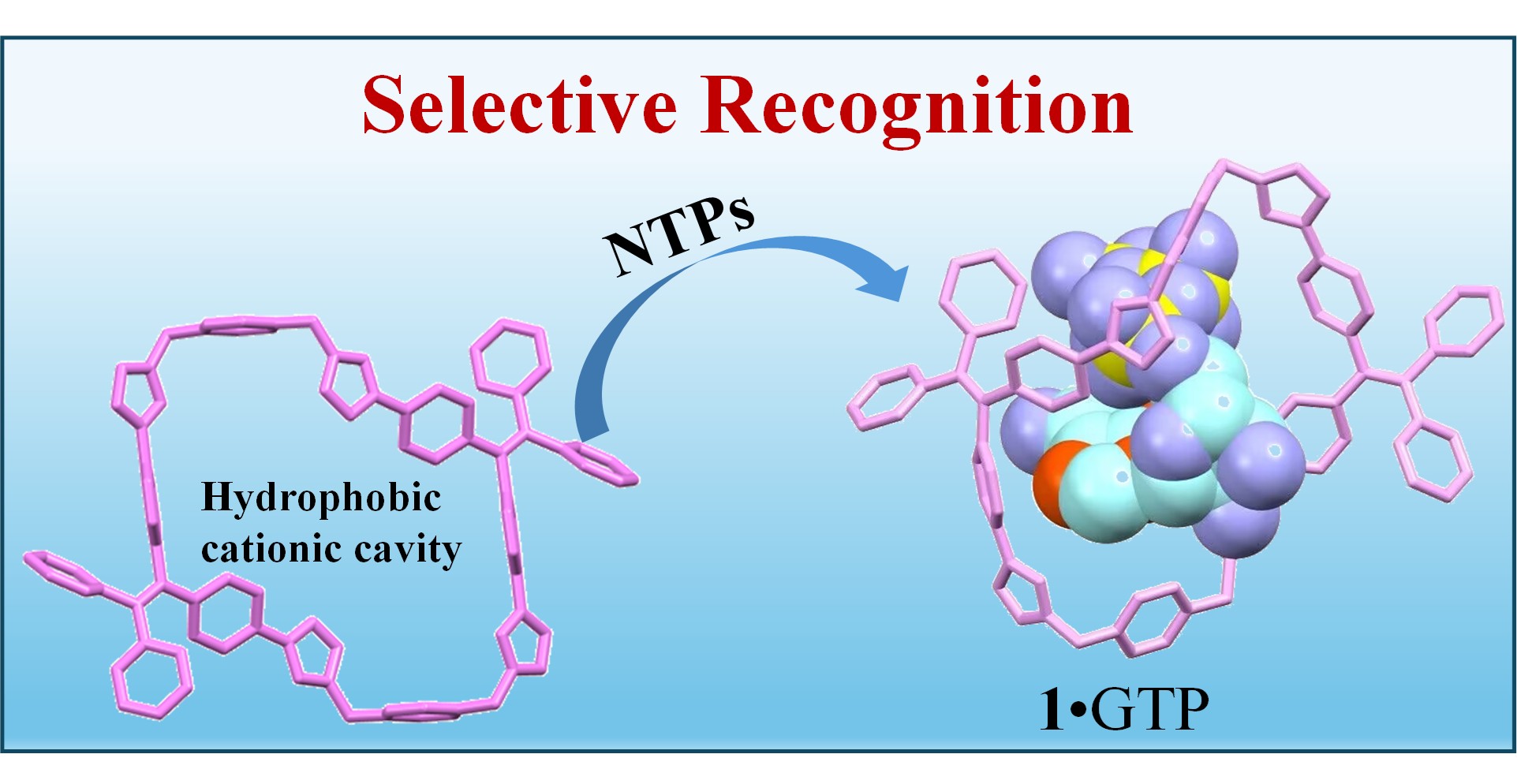
59. Wang, P.; Li, Y.; Zhao, L.; Yang, Y.; Kang, X.; Yang, T.; Cao, F.; Cheng, L.;* Cao, L.,* Selective Recognition of Nucleoside Triphosphates in Water by Tetraphenylethene-Based Tetraimidazolium Cyclophanes. Chin. J. Chem. 2025, 43, 1173-1180.
https://doi.org/10.1002/cjoc.202401247
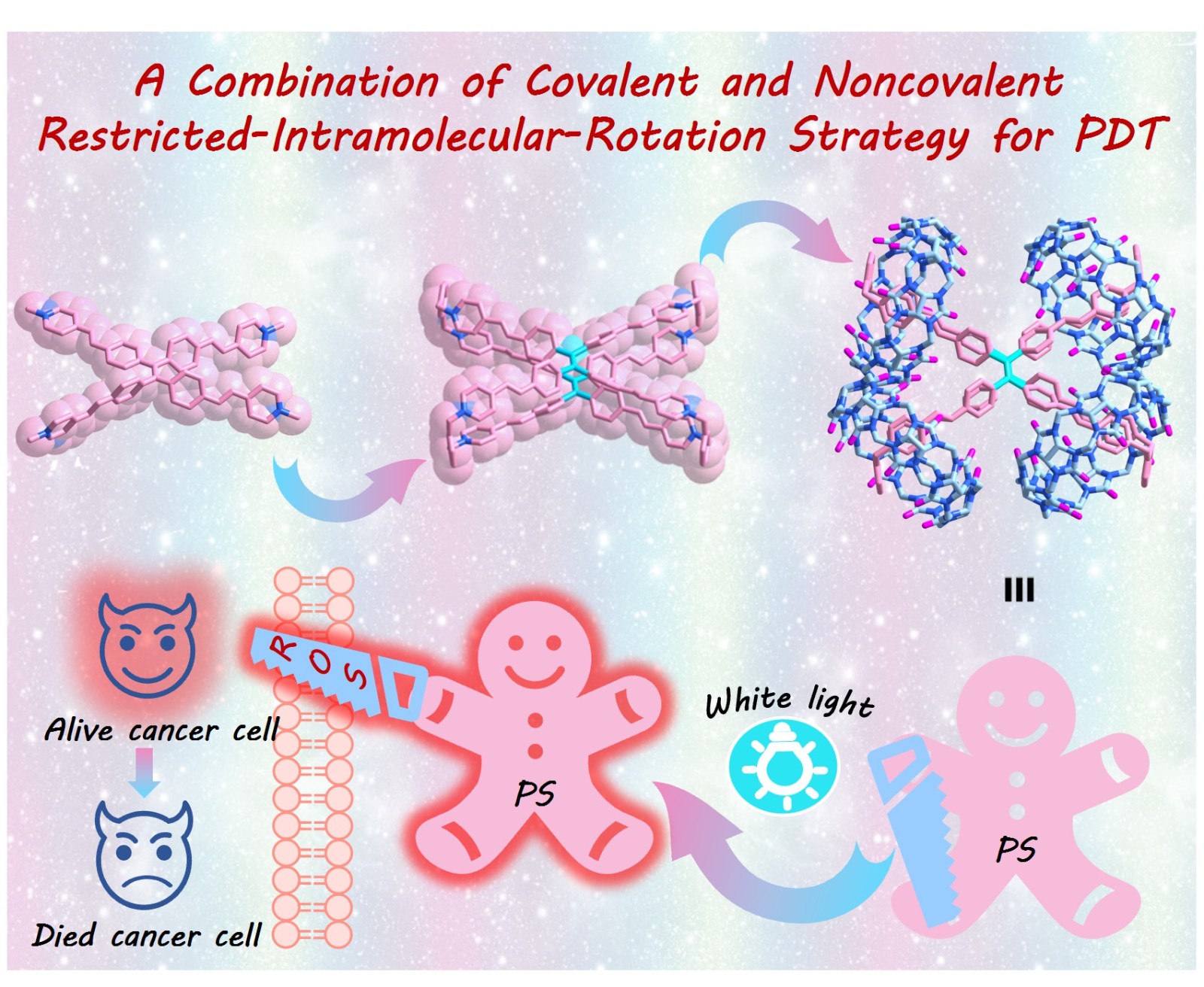
58. Li, Q.; Zhang, P.; Wang, P.; Yan, C.; Wang, K.; Yang, W.; Dang, D.;* Cao, L.,* A Combination of Covalent and Noncovalent Restricted-Intramolecular-Rotation Strategy for Supramolecular AIE-Type Photosensitizer toward Photodynamic Therapy. Aggregate, 2025, 6, e676.
https://onlinelibrary.wiley.com/doi/epdf/10.1002/agt2.676
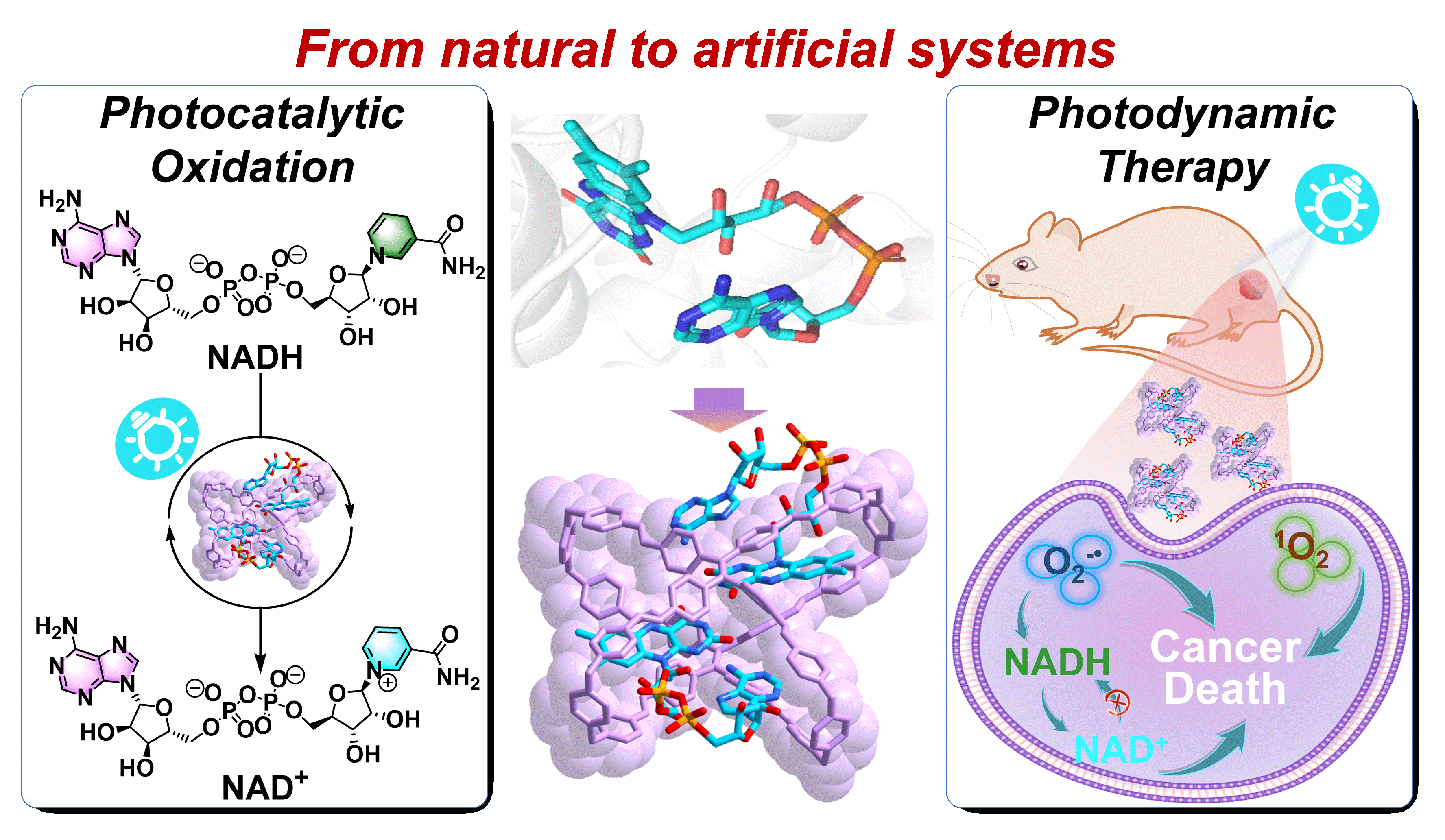
57. Li, Q.; Yan, C.; Zhang, P.; Wang, P.; Wang, K.; Yang, W.; Cheng, L.; Dang, D.; Cao, L.,* Tetraphenylethene-Based Molecular Cage with Coenzyme FAD: Conformationally Isomeric Complexation toward Photocatalysis-Assisted Photodynamic Therapy. J. Am. Chem. Soc. 2024, 146, 30933-30946.
https://pubs.acs.org/doi/10.1021/jacs.4c09508

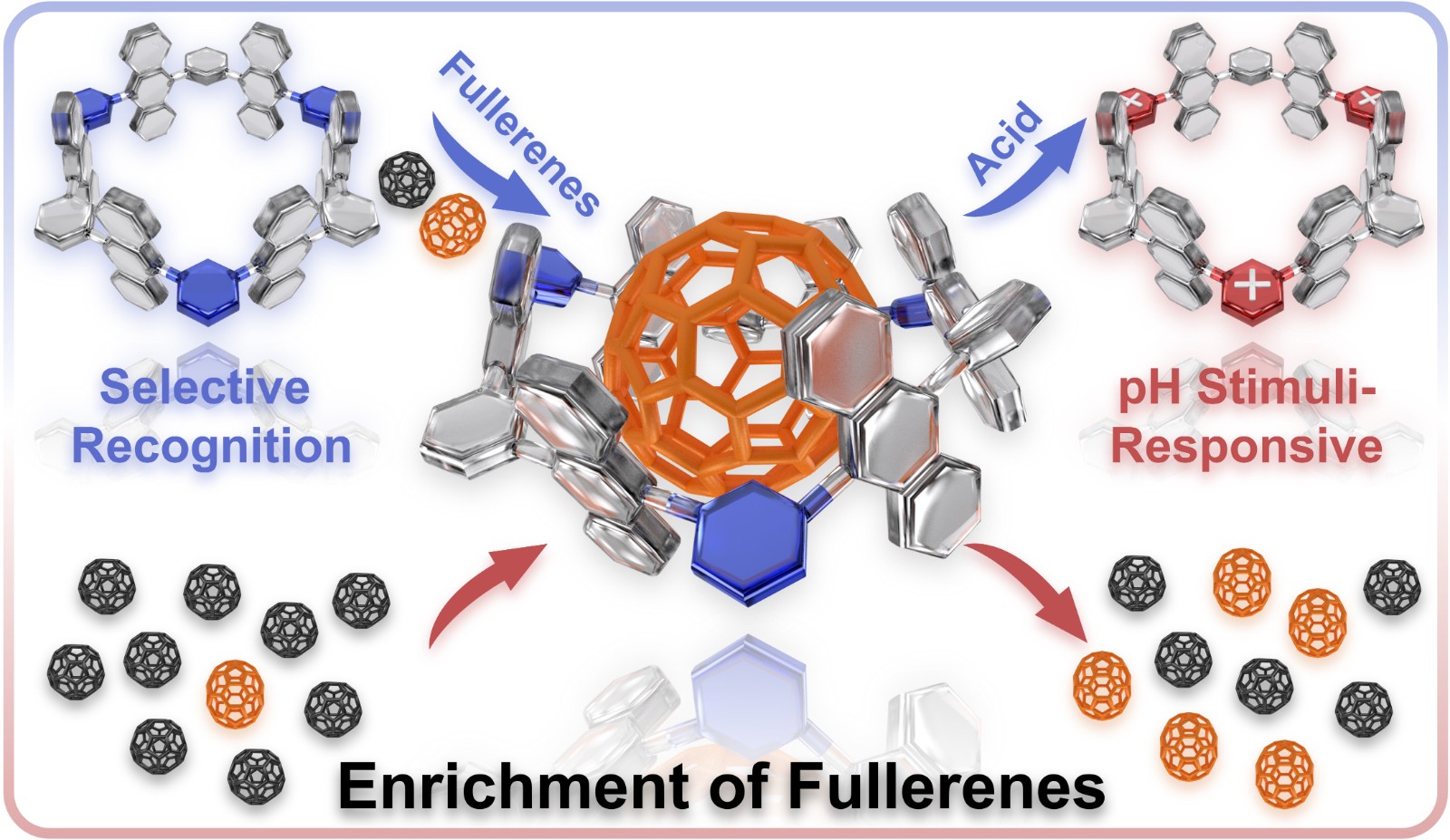
56. Nian, H.; Wang, S.-M.; Wang, Y.-F.; Zheng, Y.-T.; Zheng, L.-S.; Wang, X.; Yang, L.-P.;* Jiang, W.;* Cao, L.,* Selective Recognition and Enrichment of C70 over C60 Using an Anthracene-Based Nanotube. Chem. Sci. 2024, 15, 10214-10220.
https://pubs.rsc.org/en/content/articlelanding/2024/sc/d4sc02814g
55. Yang, T.; Duan, H.; Nian, H.; Wang, P.; Yan, C.; Cao, F.; Li, Q.; Cao, L.* Unraveling the Structure-Chirality Sensing Relationship between Achiral Anthracene-Based Tetracationic Nanotubes and Nucleosides in Aqueous Host-Guest Complexation. Biosens. Bioelectron. 2024, 258, 116342.
https://www.sciencedirect.com/science/article/pii/S0956566324003476
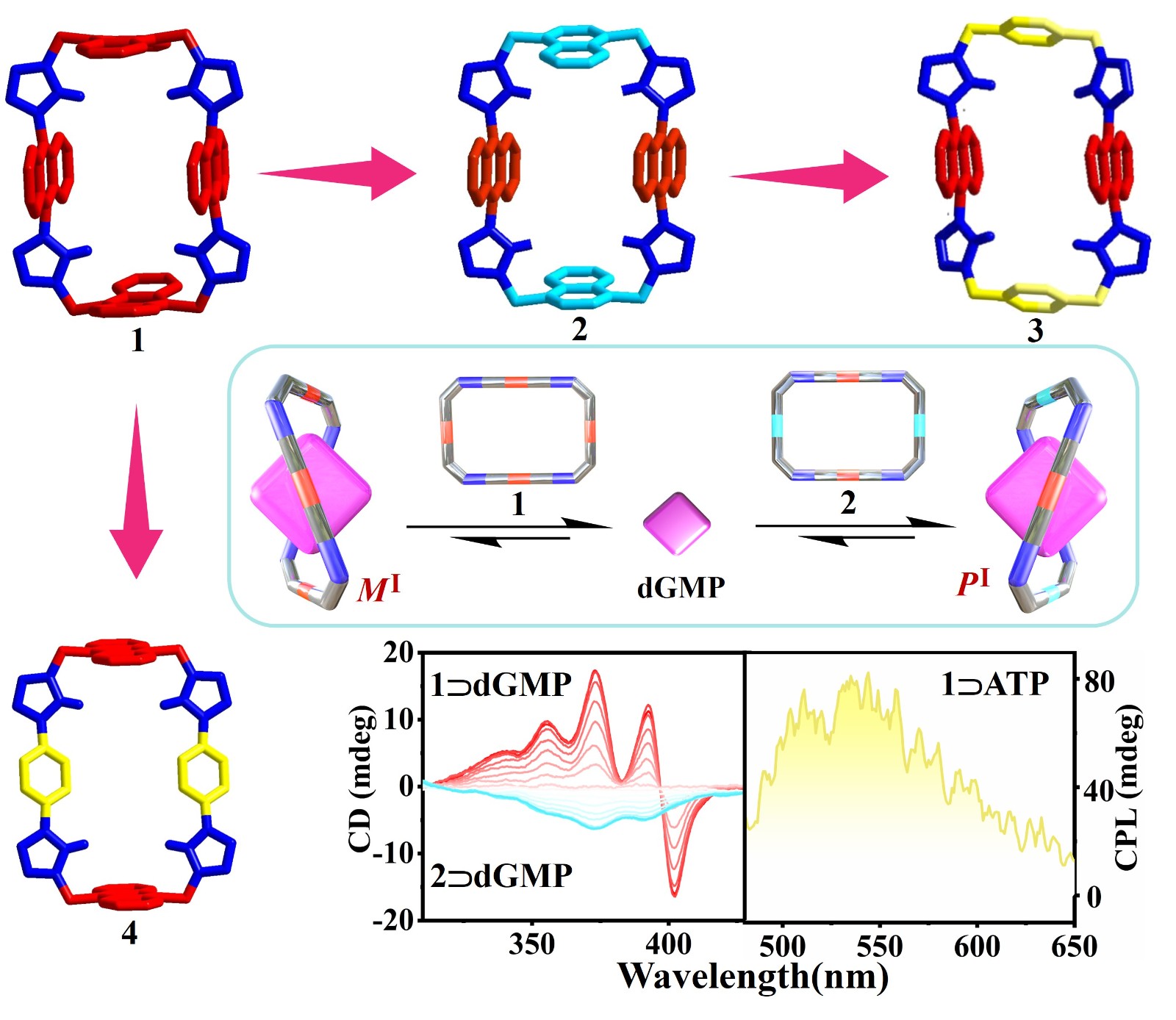
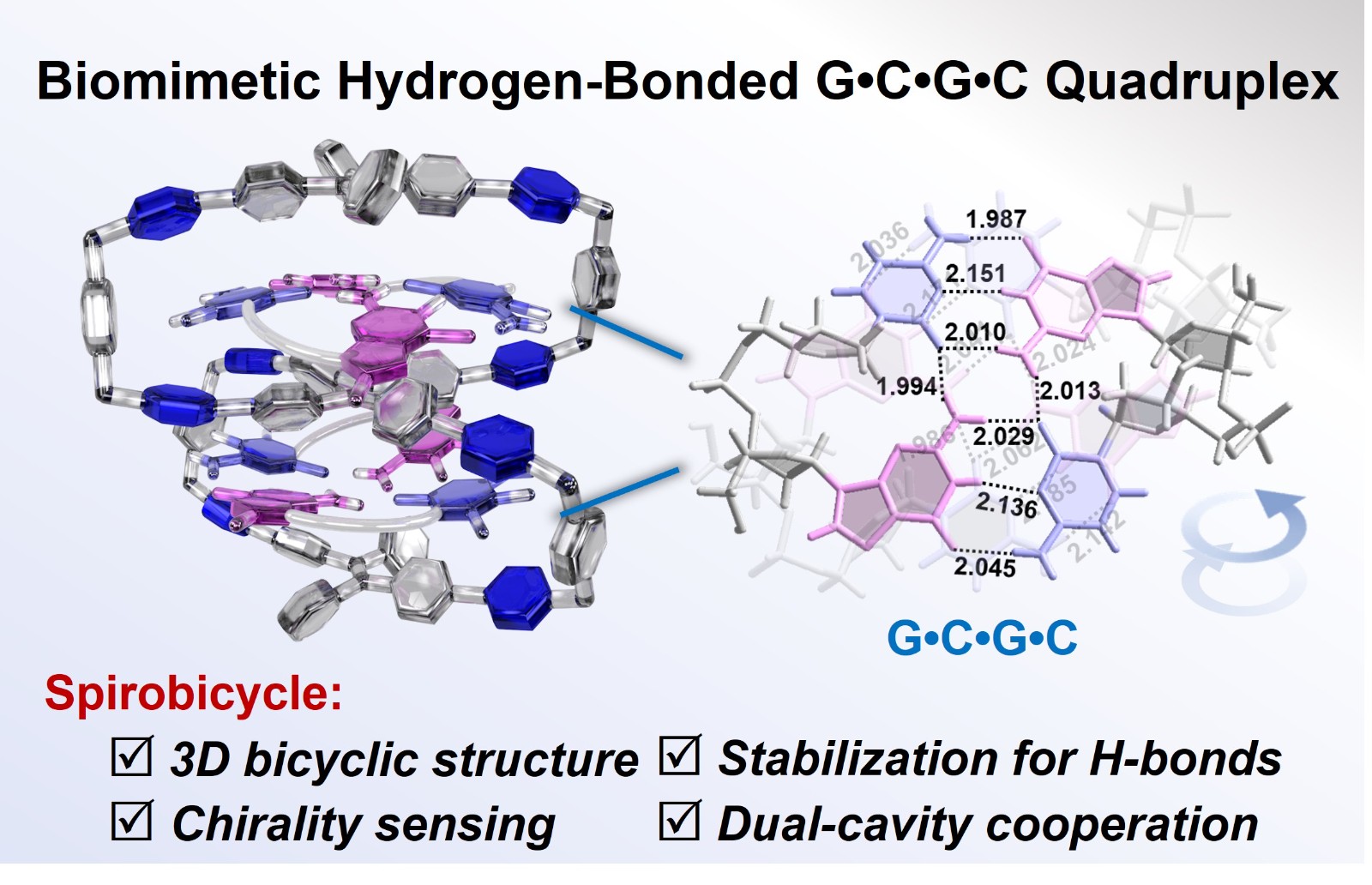
54. Zhao, L.; Cheng L.; Yang, Y.; Wang, P.; Tian, P.; Yang, T.; Nian, H.; Cao, L.* Biomimetic Hydrogen-Bonded G•C•G•C Quadruplex within a Tetraphenylethene-Based Octacationic Spirobicycle in Water. Angew. Chem. Int. Ed. 2024, e202405150. (Very Important Paper)
https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.202405150
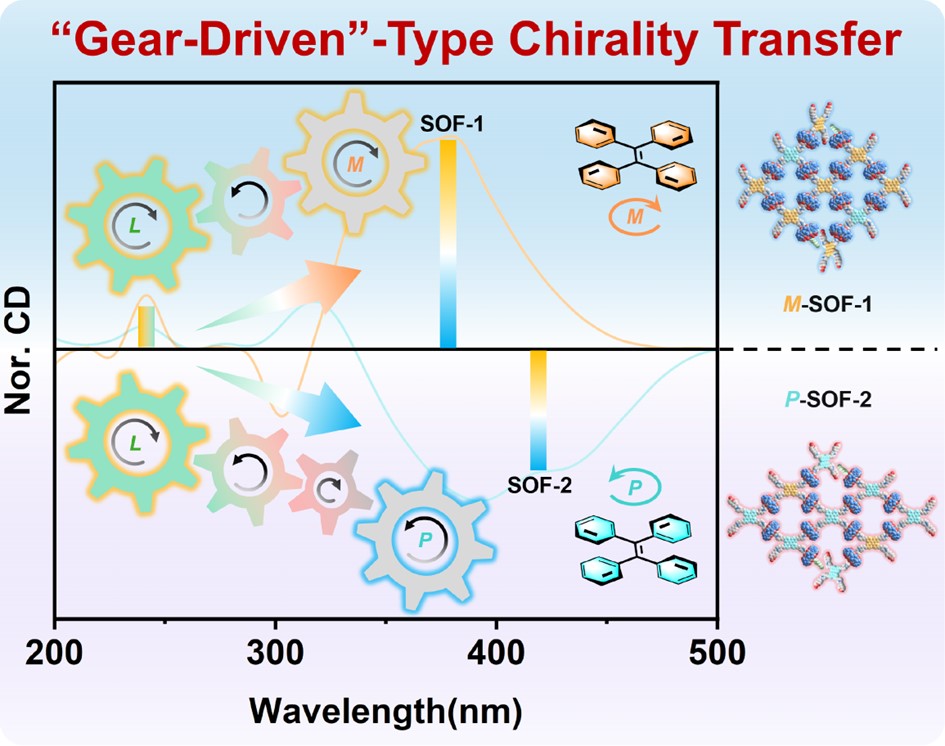
53. Yan, C.; Li, Q.; Wang, K.; Yang, W.; Han, J.; Li, Y.; Dong, Y.; Chu, D.; Cheng, L.; Cao, L.* “Gear-Driven”-Type Chirality Transfer of Tetraphenylethene-Based Supramolecular Organic Frameworks for Peptides in Water. Chem. Sci. 2024, 15, 3758-3766.
https://pubs.rsc.org/en/content/articlelanding/2024/sc/d3sc06349f
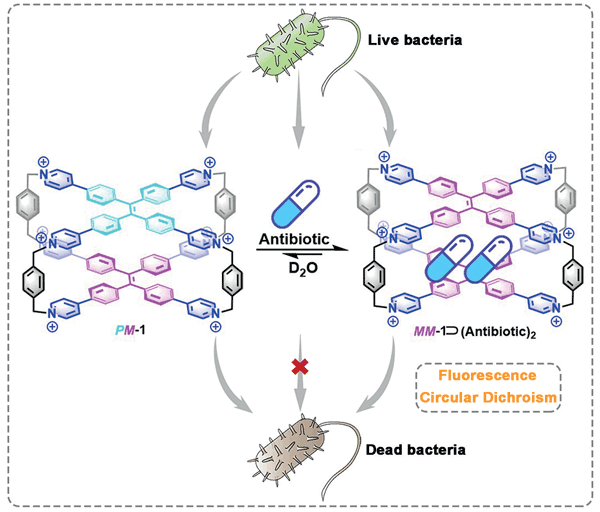
52. Dong, Y.; Cheng, L.*; Duan, Y.; Xu, H.; Dong, R.; Guo, B.; Cao, L.* Dual Responses of Fluorescence and Circular Dichroism for Antibiotics by a Cationic Cage in Water. Synlett. 2023, 35, 109-112.
https://www.thieme-connect.com/products/ejournals/abstract/10.1055/a-2109-0055
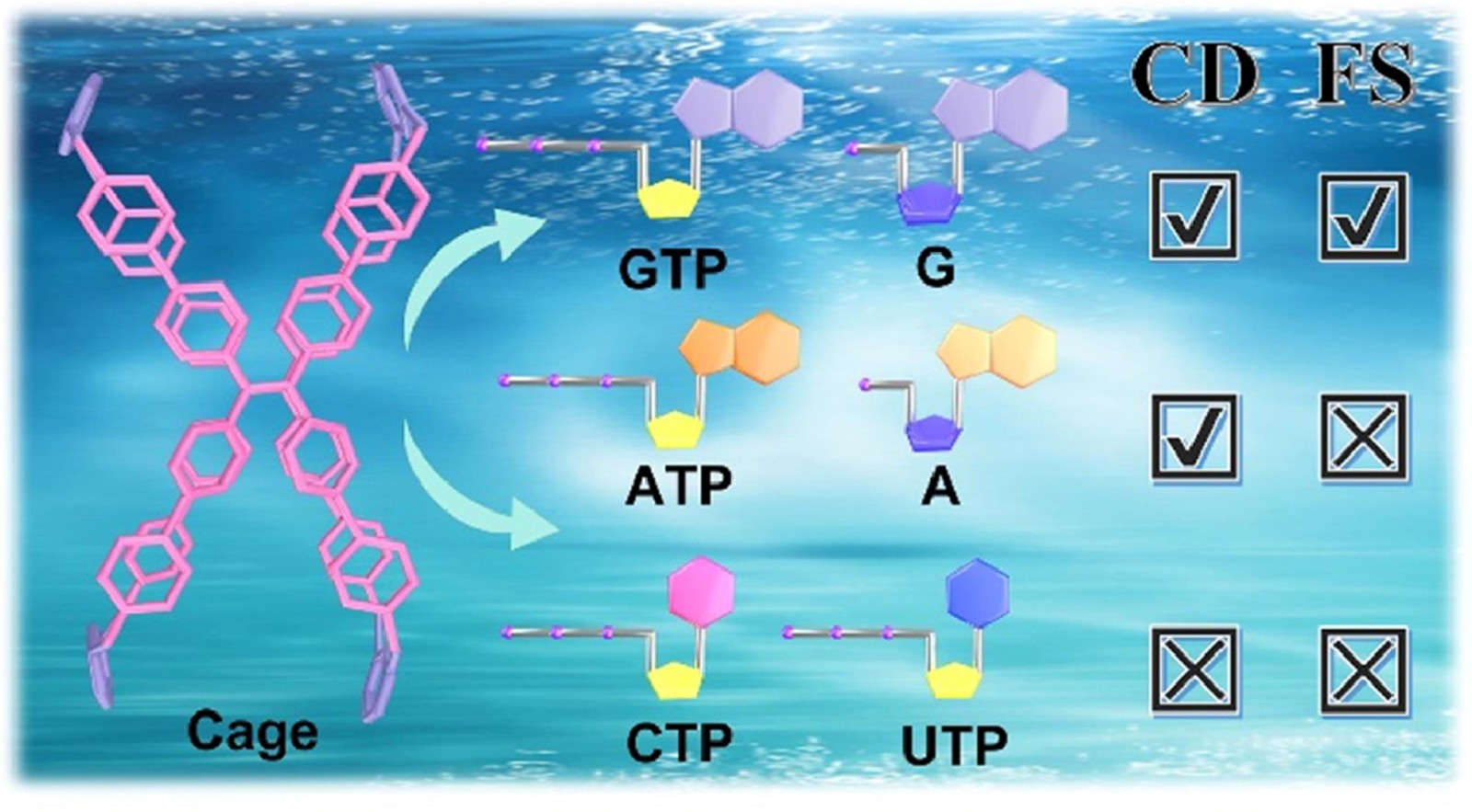
51. Duan, H.; Yang, T.; Li, Q.; Cao, F.; Wang, P.; Cao, L.* Recognition and chirality sensing of guanosine-containing nucleotides by an achiral tetraphenylethene-based octacationic cage in water. Chin. Chem. Lett. 2023, 108878.
https://www.sciencedirect.com/science/article/abs/pii/S1001841723006976
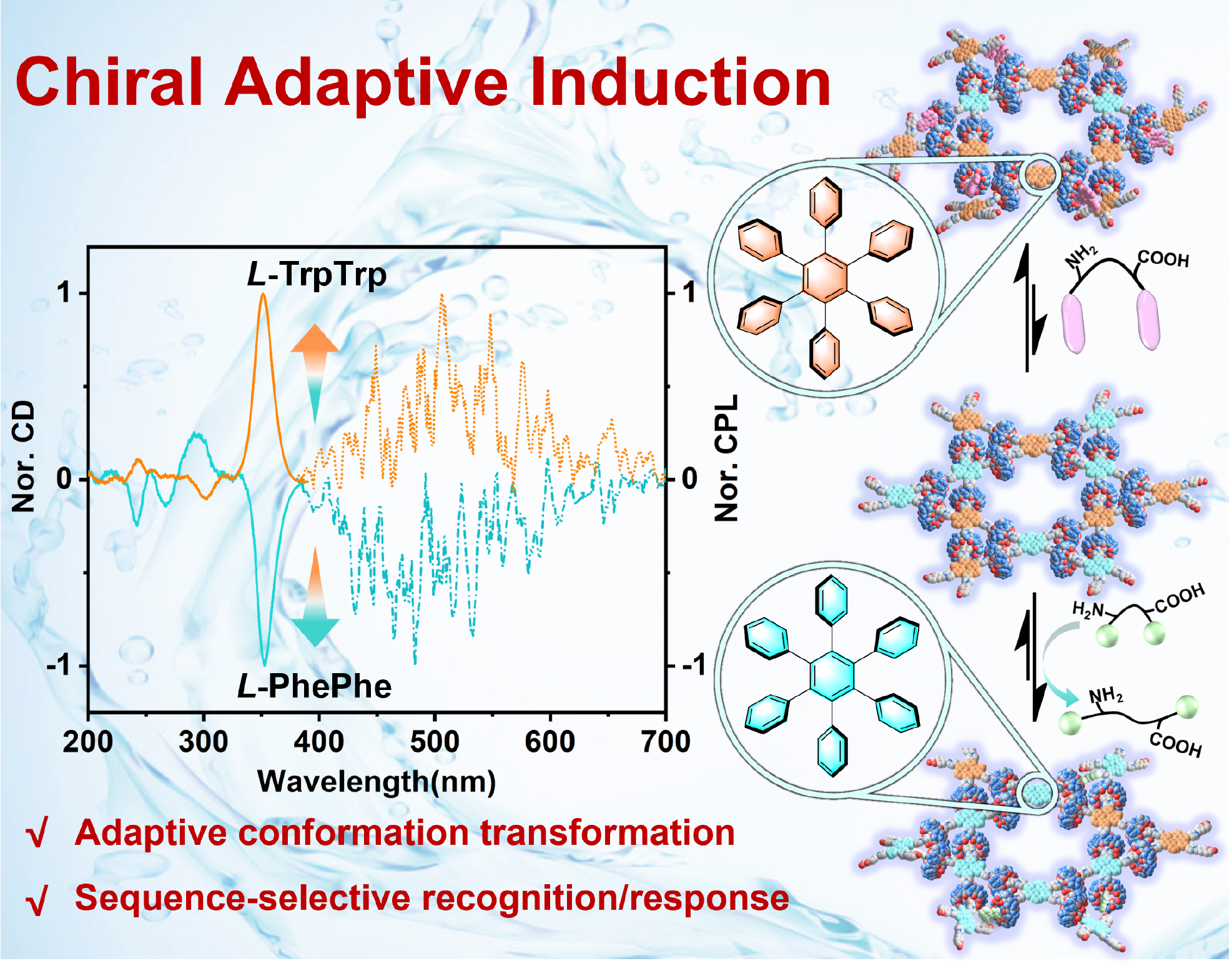
50. Yan, C.; Li, Q.; Miao, X.; Zhao, Y.; Li, Y.; Wang, P.; Wang, K.; Duan, H.; Zhang, L.; Cao, L.* Chiral Adaptive Induction of an Achiral Cucurbit[8]uril-Based Supramolecular Organic Framework by Dipeptides in Water. Angew. Chem. Int. Ed. 2023, 62, e202308029. (HOT PAPER)
https://onlinelibrary.wiley.com/doi/10.1002/anie.202308029
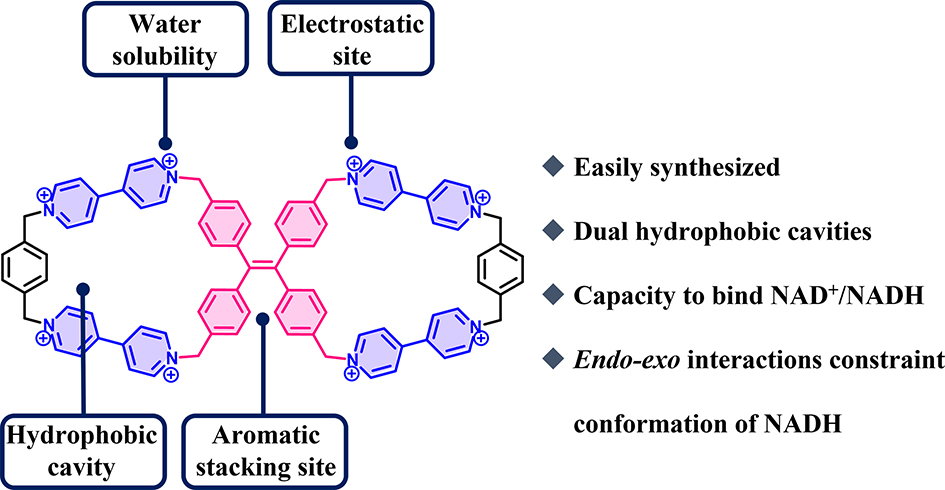
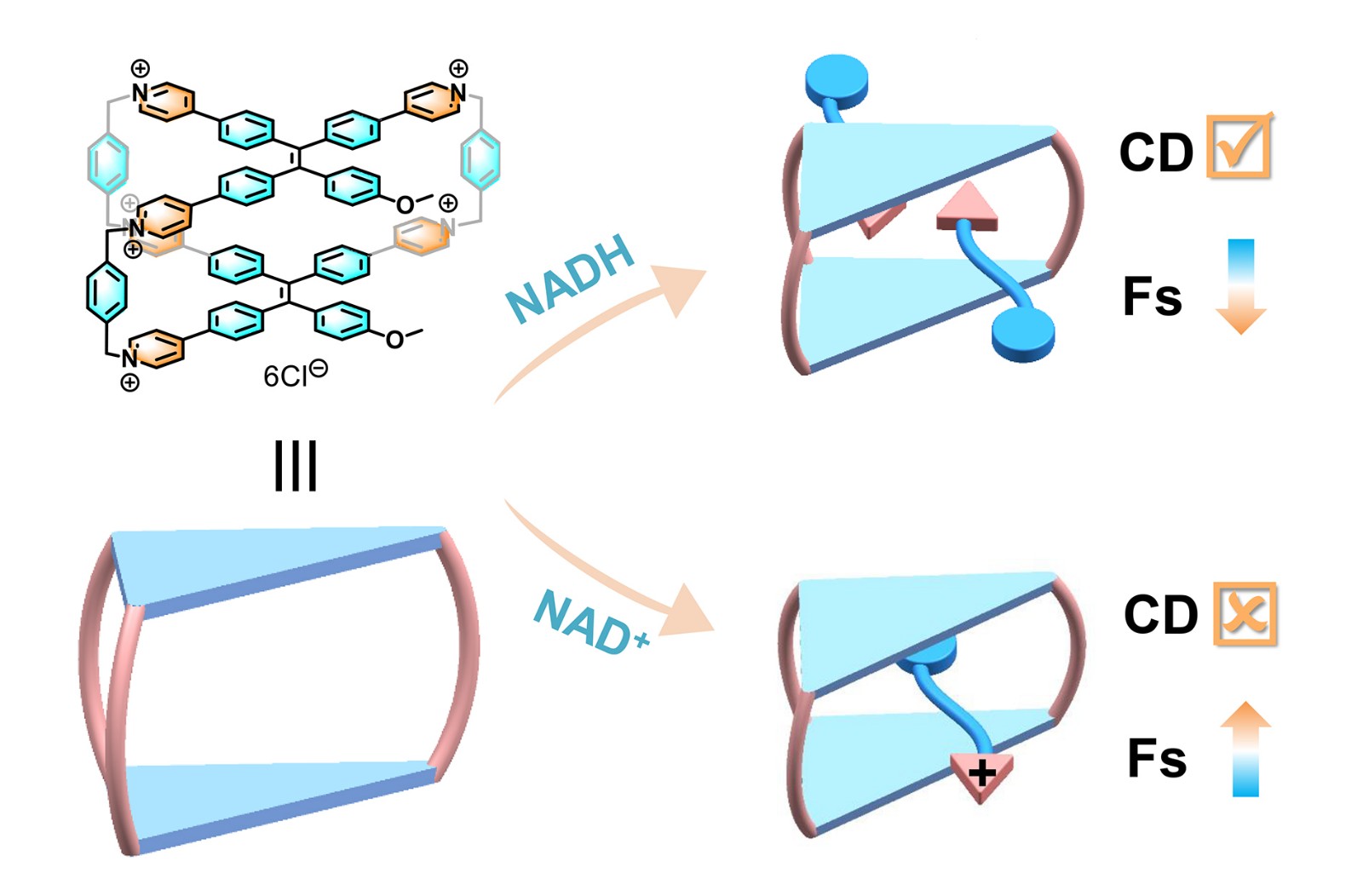
49. Wang, L.; Guo. Z.; Cheng, L.*; Nian, H.; Yao, N.; Zhao, Y.; Liu, K.*; Cao, L.* Tetraphenylethene-linked octacationic dicyclophanes with enhanced recognition of NADH over NAD+ in water. Dyes and Pigments, 2023, 216, 111364.
https://www.sciencedirect.com/science/article/pii/S0143720823002905
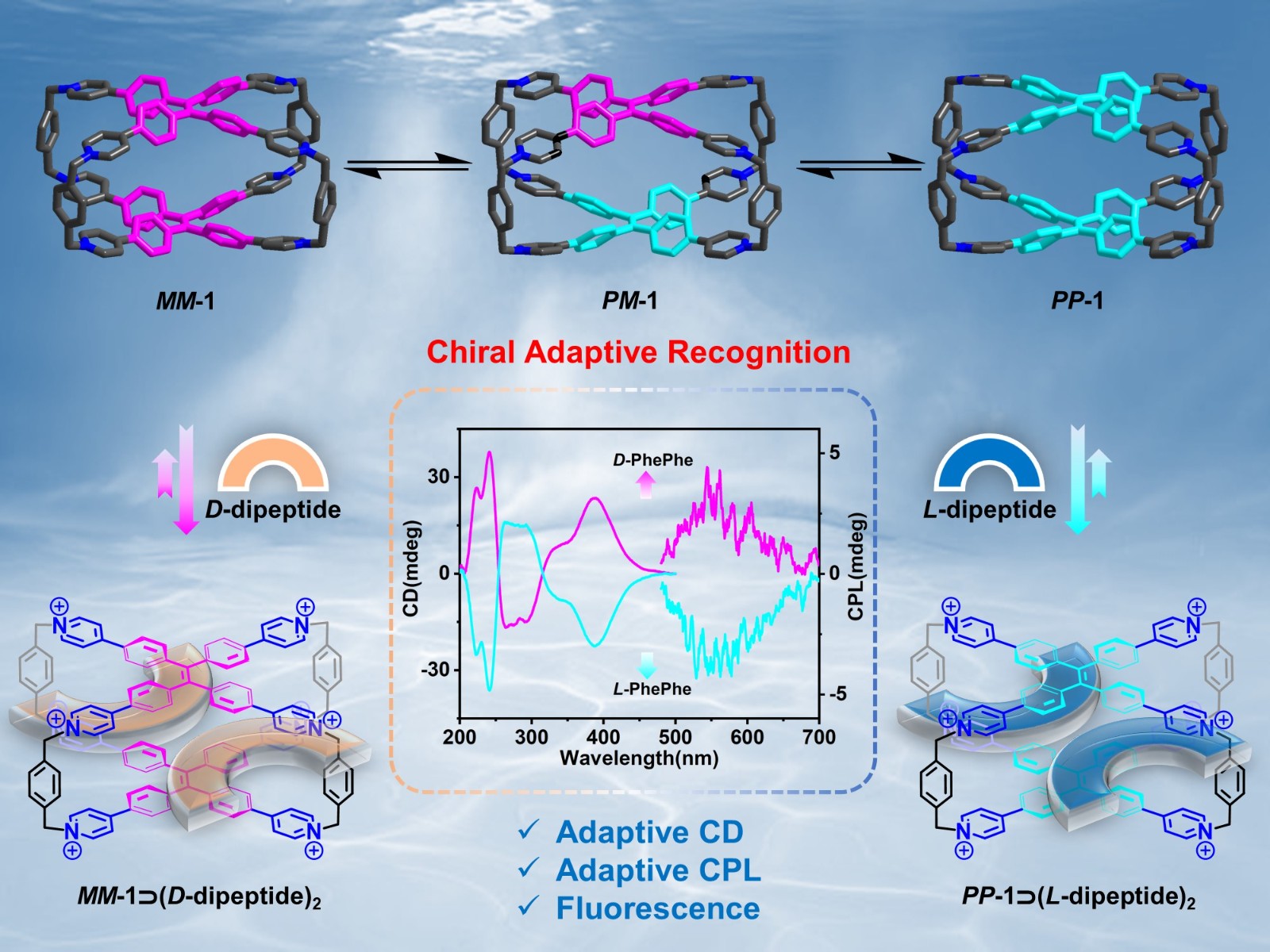
48. Cheng, L.; Tian, P.; Duan, H.; Li, Q.; Song, X.; Li, A.; Cao, L.* Chiral Adaptive Recognition with Sequence Specificity of Aromatic Dipeptides in Aqueous Solution by an Achiral Cage. Chem. Sci. 2023, 14, 833-842.
https://pubs.rsc.org/en/content/articlelanding/2023/sc/d2sc05854e
47. Cao, F.; Duan, H.; Li, Q.; Cao, L.* A Tetraphenylethene-Based Hexacationic Molecular Cage with an Open Cavity. Chem. Commun. 2022, 58, 13389-13392.
https://pubs.rsc.org/en/content/articlelanding/2022/cc/d2cc05153b
46. Duan, Y.; Wang, J.; Cheng, L.; Duan, H.; Tian, P.; Zhang, Y.*; Cao, L.* Fluorescent, Chirality-Responsive, and Water-Soluble Cage as a Multifunctional Molecular Container for Drug Delivery. Org. & Biomole. Chem. 2022, 20, 3998-4005.
https://pubs.rsc.org/en/content/articlelanding/2022/ob/d2ob00520d
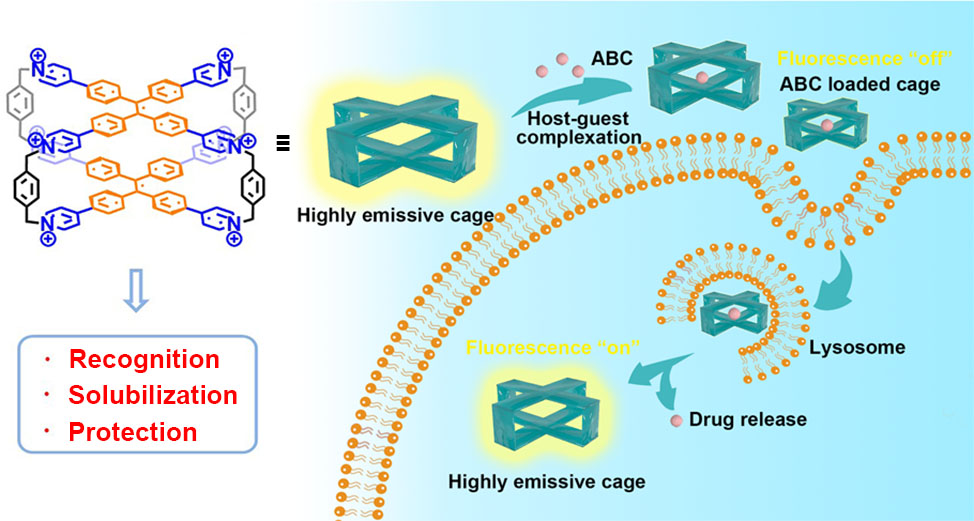
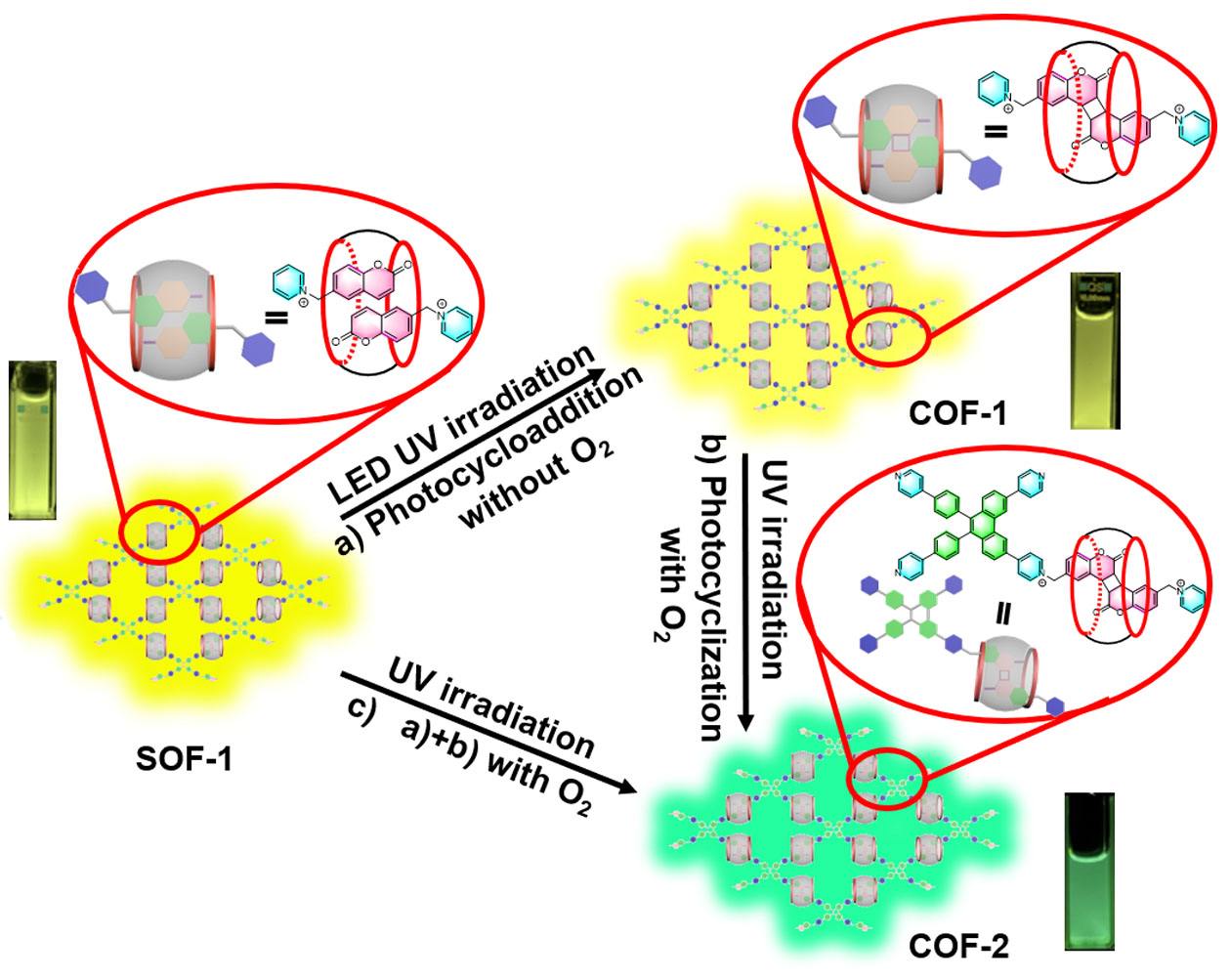
45. Li, Y.; Yan, C.; Li, Q.; Cao, L.* Successive Construction of Cucurbit[8]uril-Based Covalent Organic Frameworks from a Supramolecular Organic Framework through Photochemical Reactions in Water. Sci. China Chem. 2022, 65, 1279-1285.
http://engine.scichina.com/doi/10.1007/s11426-022-1231-5
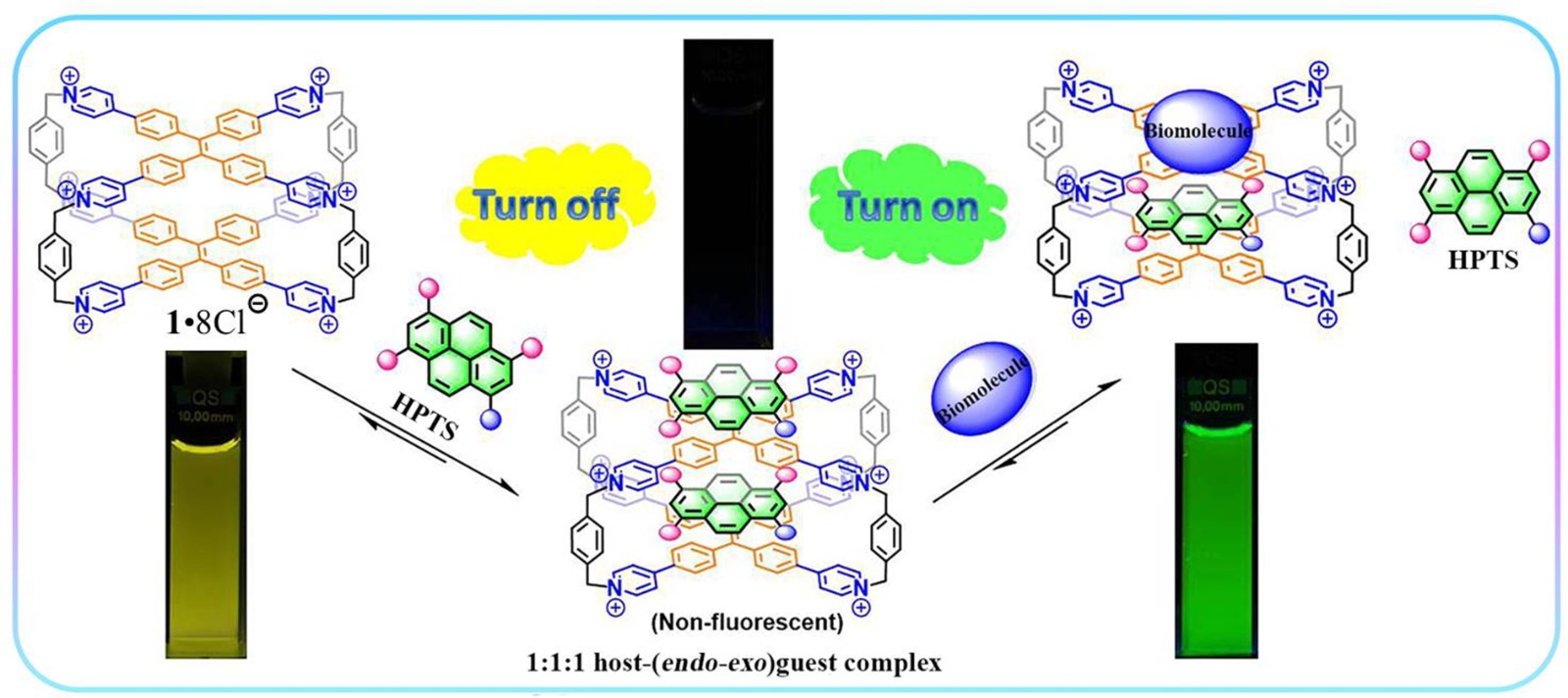
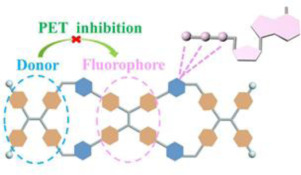
44. Duan, H.; Cao, F.; Zhang, M.; Gao, M.; Cao, L.* On-Off-On Fluorescence Detection for Biomolecules by a Fluorescent Cage through Host-Guest Complexation in Water. Chin. Chem. Lett. 2022, 33, 2459-2463.
https://www.sciencedirect.com/science/article/pii/S1001841721009426
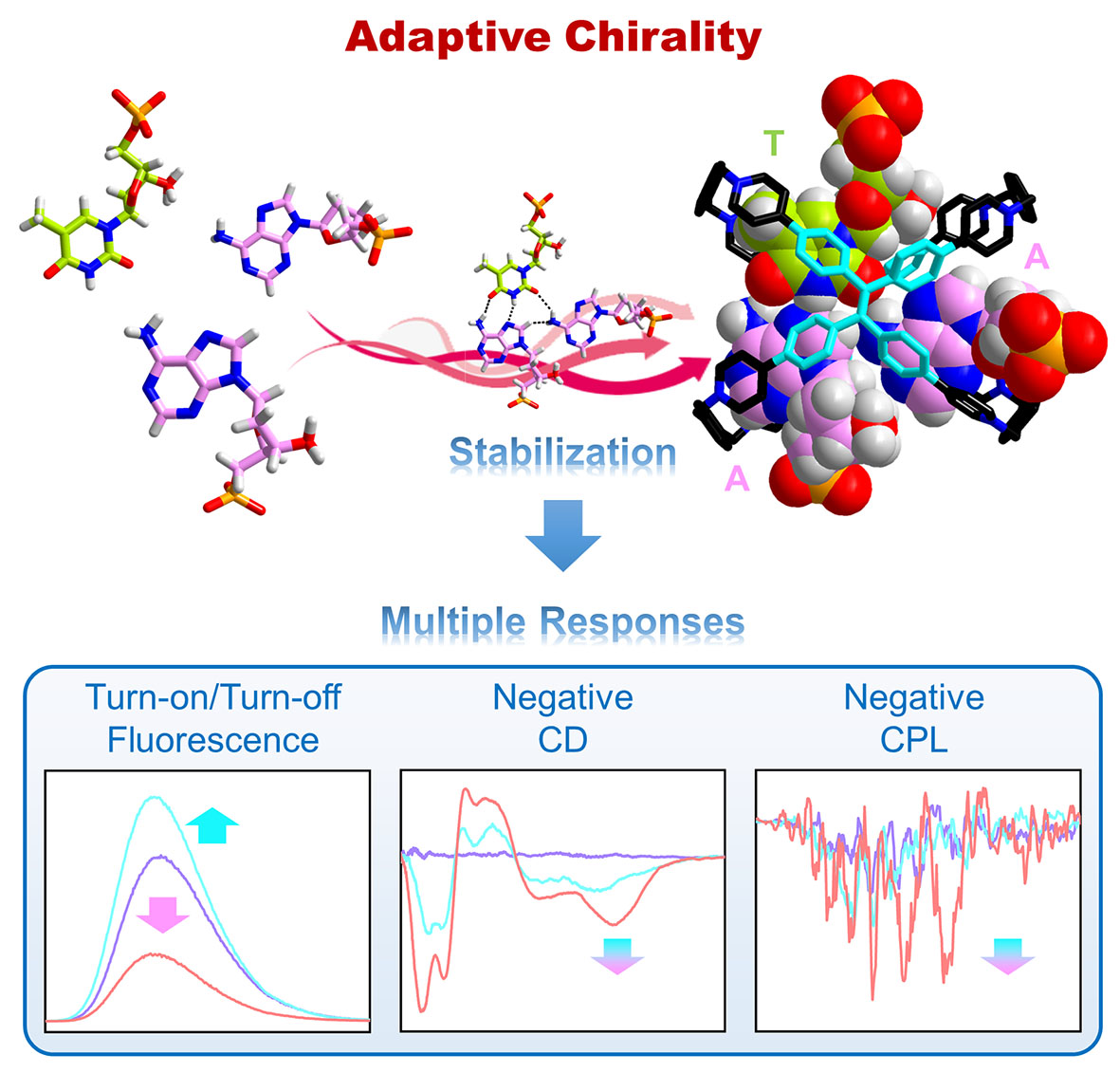
43. Cheng, L.; Tian, P.; Li, Q.; Li, A.; Cao, L.* Stabilization and Multiple-Responsive Recognition of Natural Base Pairs in Water by a Cationic Cage. CCS Chem. 2022, 4, 2914-2920.
https://www.chinesechemsoc.org/doi/10.31635/ccschem.021.202101584
42. Wang, P.; Liu, K.; Ma, H.; Nian, H.; Li, Y.; Li, Q.; Cheng, L.; Cao, L.* Synthesis and Aqueous Anion Recognition of Imidazolium-Based Nonacationic Cup. Chem. Commun. 2021, 57, 13377-13380.
https://pubs.rsc.org/en/content/articlehtml/2021/CC/D1CC05603D
41. Nian, H.; Cheng, L.; Wang, L.; Zhang, H.; Wang, P.; Li, Y.; Cao, L.* Hierarchical Two-Level Supramolecular Chirality of an Achiral Anthracene-Based Tetracationic Nanotube in Water. Angew. Chem. Int. Ed. 2021, 60, 15354-15358.
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/anie.202105593
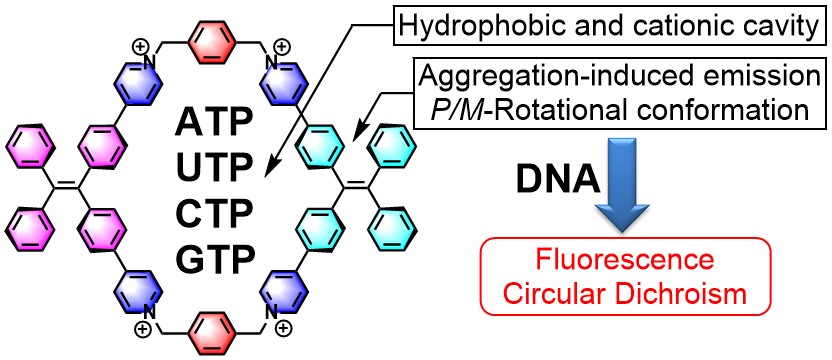
40. Qin, C.; Li, Y.; Li, Q.; Yan, C.; Cao, L.* Aggregation-Induced Emission and Self-Assembly of Functional Tetraphenylethene-Based Tetracationic Dicyclophanes for Selective Detection of ATP in Water. Chin. Chem. Lett. 2021, 32, 3531-3534.
https://www.sciencedirect.com/science/article/pii/S1001841721003107
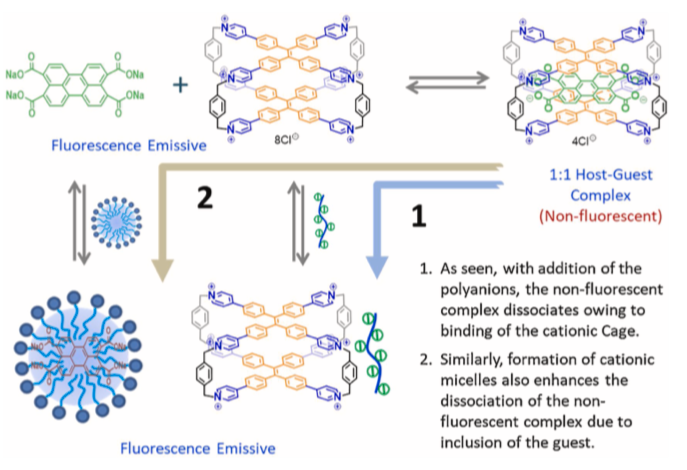
39. Xu, W.; Duan, H.; Chang, X.; Wang, G.; Hu, D.; Wang, Z.; Cao, L.*; Fang, Y.* Polyanion and Anionic Surface Monitoring in Aqueous Medium Enabled by an Ionic Host-Guest Complex. Sensors and Actuators B: Chemical, 2021, 340, 129916.
https://www.sciencedirect.com/science/article/pii/S0925400521004858
38. Duan, H.; Cao, F.; Hao, H.; Bian, H.; Cao, L.* Efficient Photoinduced Energy and Electron Transfers in a Tetraphenylethene-Based Octacationic Cage through Host-Guest Complexation. ACS Appl. Mater. & Interfaces 2021, 13, 16837-16845.
https://pubs.acs.org/doi/10.1021/acsami.1c01867
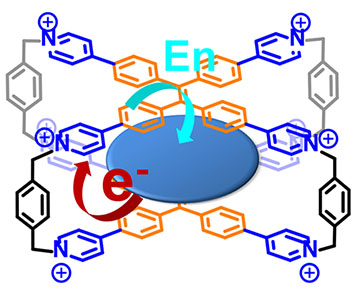
37. Zhang, H.; Cheng, L.; Nian, H.; Du, J.; Chen, T.; Cao, L.* Adaptive Chirality of Achiral Tetraphenylethene-Based Tetracationic Cyclophanes with Dual Responses of Fluorescence and Circular Dichroism in Water. Chem. Commun. 2021, 57, 3135-3138.
https://doi.org/10.1039/d1cc00303h
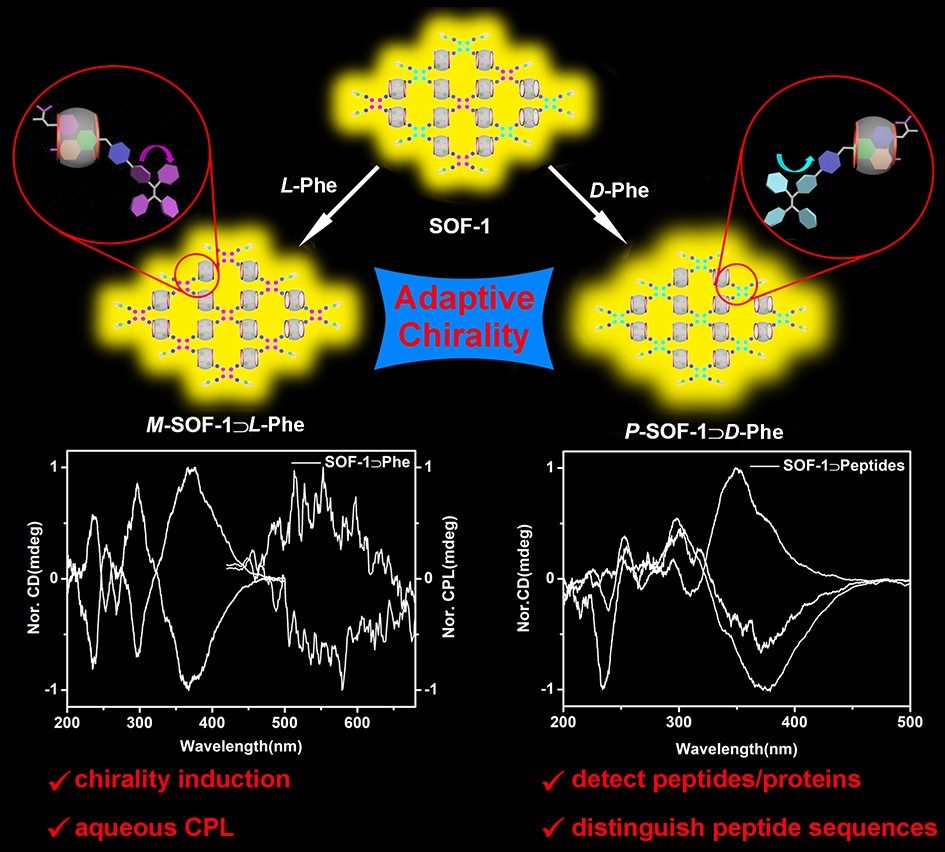
36. Li, Y.; Li, Q.; Miao, X.; Qin, C.; Chu, D.; Cao, L.* Adaptive Chirality of an Achiral Cucurbit[8]uril-Based Supramolecular Organic Framework for Chirality Induction in Water. Angew. Chem. Int. Ed. 2021, 60, 6744-6751.
https://doi.org/10.1002/anie.202012681
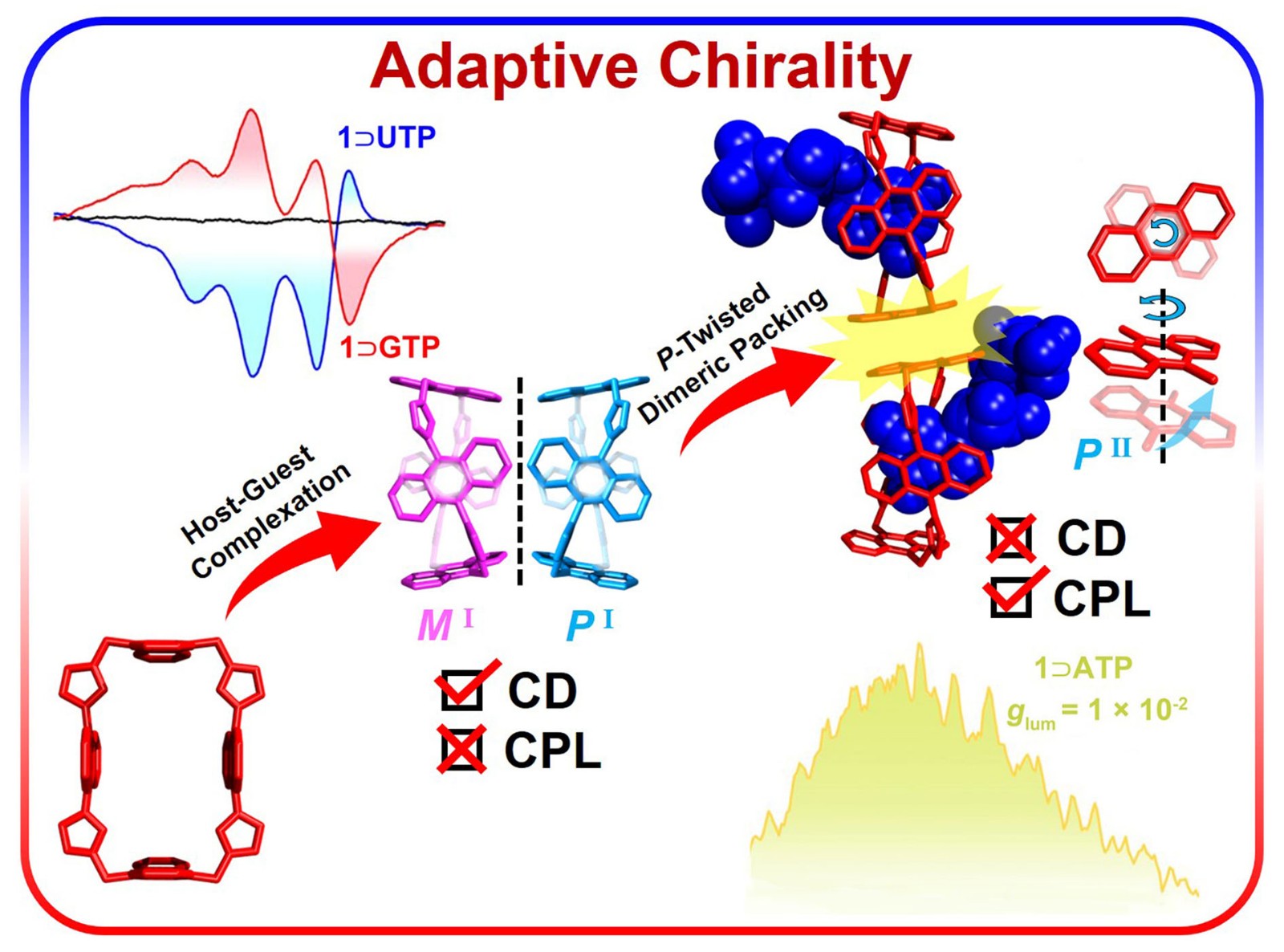
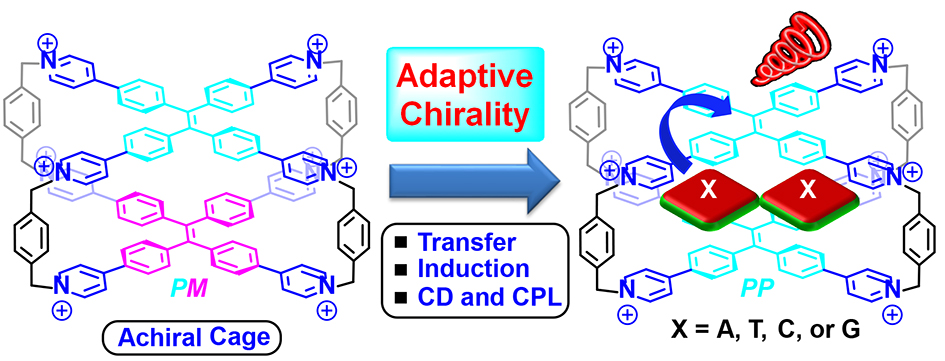
35. Cheng, L.; Liu, K.; Duan, Y.; Duan, H.; Li, Y.; Gao, M.; Cao, L.* Adaptive Chirality of an Achiral Cage: Chirality Transfer, Induction, and Circularly Polarized Luminescence Through Aqueous Host-Guest Complexation. CCS Chem. 2021, 3, 2749-2763.
https://www.chinesechemsoc.org/doi/abs/10.31635/ccschem.020.202000509
34. Duan, H.; Li, Y.; Li, Q.; Wang, P.; Liu, X.; Cheng, L.; Yu, Y.; Cao, L.* Host-Guest Recognition and Fluorescence of a Tetraphenylethene-Based Octacationic Cage. Angew. Chem., Int. Ed. 2020, 59, 10101-10110.
https://doi.org/10.1002/anie.201912730
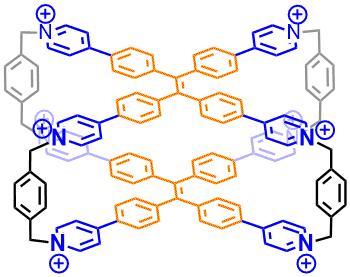
33. Wang, P.; Miao, X.; Meng, Y.; Wang, Q.; Wang, J.*; Duan, H.; Li, Y.; Li, C.; Liu, J.; Cao, L.* Tetraphenylethene-Based Supramolecular Coordination Frameworks with Aggregation-Induced Emission for Artificial Light-Harvesting System. ACS Appl. Mater. & Interfaces 2020, 12, 22630-22639.
https://pubs.acs.org/doi/pdf/10.1021/acsami.0c04917
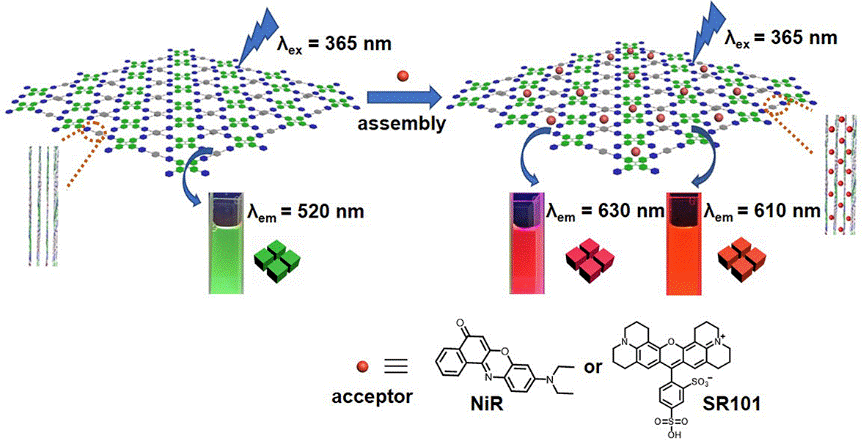
32. Li, Y.; Qin, C.; Li, Q.; Wang, P.; Miao, X.; Jin, H.; Ao, W.; Cao, L.* Supramolecular Organic Frameworks with Controllable Shape and Aggregation-Induced Emission for Tunable Luminescent Materials Through Aqueous Host-Guest Complexation. Adv. Opt. Mater. 2020, 1902154.
https://onlinelibrary.wiley.com/doi/abs/10.1002/adom.201902154
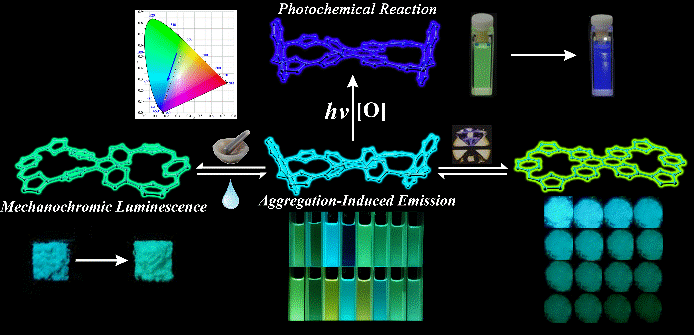
31. Nian, H.; Li, A.; Li, Y.; Cheng, L.; Wang, L.; Xu, W.; Cao, L.* Tetraphenylethene-Based Tetracationic Dicyclophanes: Synthesis, Mechanochromic Luminescence, and Photochemical Reaction. Chem. Commun. 2020, 56, 3195-3198.
https://doi.org/10.1039/d0cc00860e
30. Li, C.; Nian, H.; Dong, Y.*; Li, Y,; Zhang, B.; Cao, L.* Tetraphenylethene-Based Platinum(II) Bis-Triangular Dicycles with Tunable Emissions. Inorg. Chem. 2020, 59, 5713-5720.
https://pubs.acs.org/doi/abs/10.1021/acs.inorgchem.0c00505
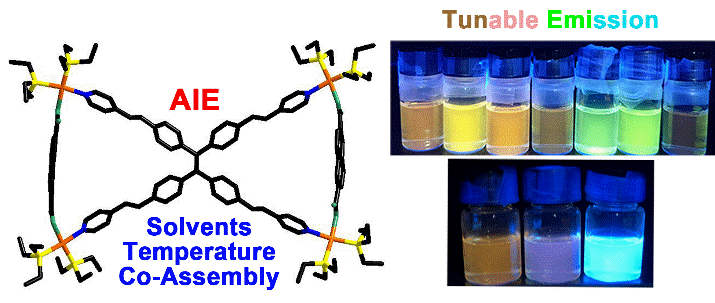
29. Li, C.; Zhang, B.; Dong, Y.*; Li, Y.; Wang, P.; Yu, Y.; Cheng, L.; Cao, L.* A Tetraphenylethene-Based Pd2L4 Metallacage with Aggregation-Induced Emission and Stimuli-Responsive Behavior. Dalton Trans. 2020, 49, 8051-8055.
https://pubs.rsc.org/en/content/articlelanding/2020/DT/D0DT00469C#!divAbstract
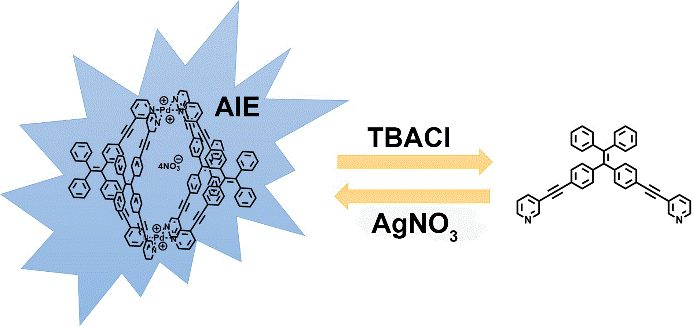
28. Li, Y.; Dong, Y.; Cheng, L.; Qin, C.; Nian, H.; Zhang, H.; Yu, Y.; Cao, L.* Aggregation-Induced Emission and Light-Harvesting Function of Tetraphenylethene-Based Tetracationic Dicyclophane. J. Am. Chem. Soc. 2019, 141, 8412-8415
https://doi.org/10.1021/jacs.9b02617
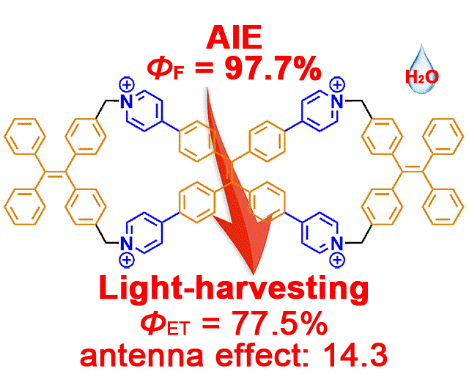

27. Cao, L.*; Wang, P.; Miao, X.; Duan, H.; Wang, H.; Dong, Y.; Ma, R.; Zhang, B.; Wu, B.; Li, X.; Stang, P. J. Diamondoid Frameworks via Supramolecular Coordination: Structural Characterization, Metallogel Formation, and Adsorption Study. Inorg. Chem. 2019, 58, 6268-6275.
https://pubs.acs.org/doi/10.1021/acs.inorgchem.9b00484
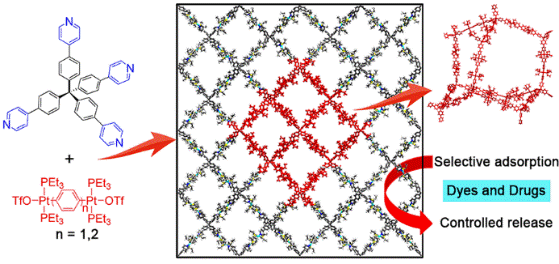
26. Cheng, L.; Zhang, H.; Dong, Y.; Zhao, Y.; Yu, Y.; Cao, L.* Tetraphenylethene-Based Tetracationic Cyclophanes and Their Selective Recognition for Amino Acids and Adenosine Derivatives in Water. Chem. Commun. 2019, 55, 2372 - 2375.
https://pubs.rsc.org/en/content/articlepdf/2019/cc/c9cc00599d
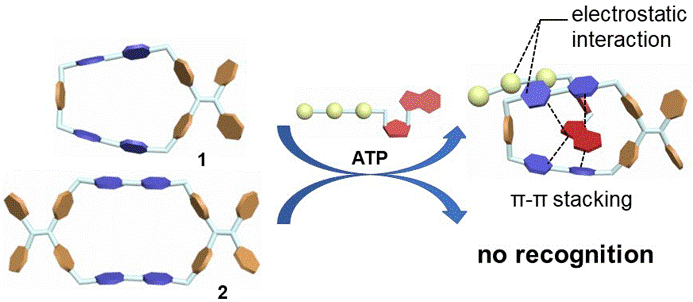
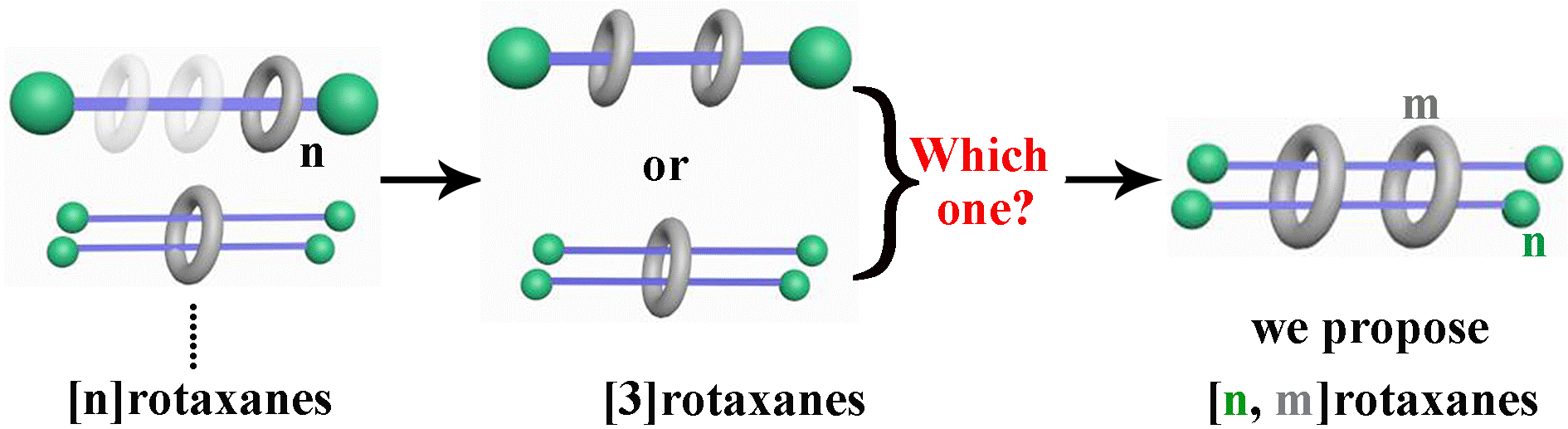
25. Zhang, B.; Dong, Y.; Li, J.; Yu, Y.; Li, C.; Cao, L.* Pseudo[n,m]rotaxanes of Cucurbit[7/8]uril and Viologen-Naphthalene Derivative: A Precise Definition of Rotaxane. Chin. J. Chem. 2019, 37, 262-275.
https://onlinelibrary.wiley.com/doi/10.1002/cjoc.201800562
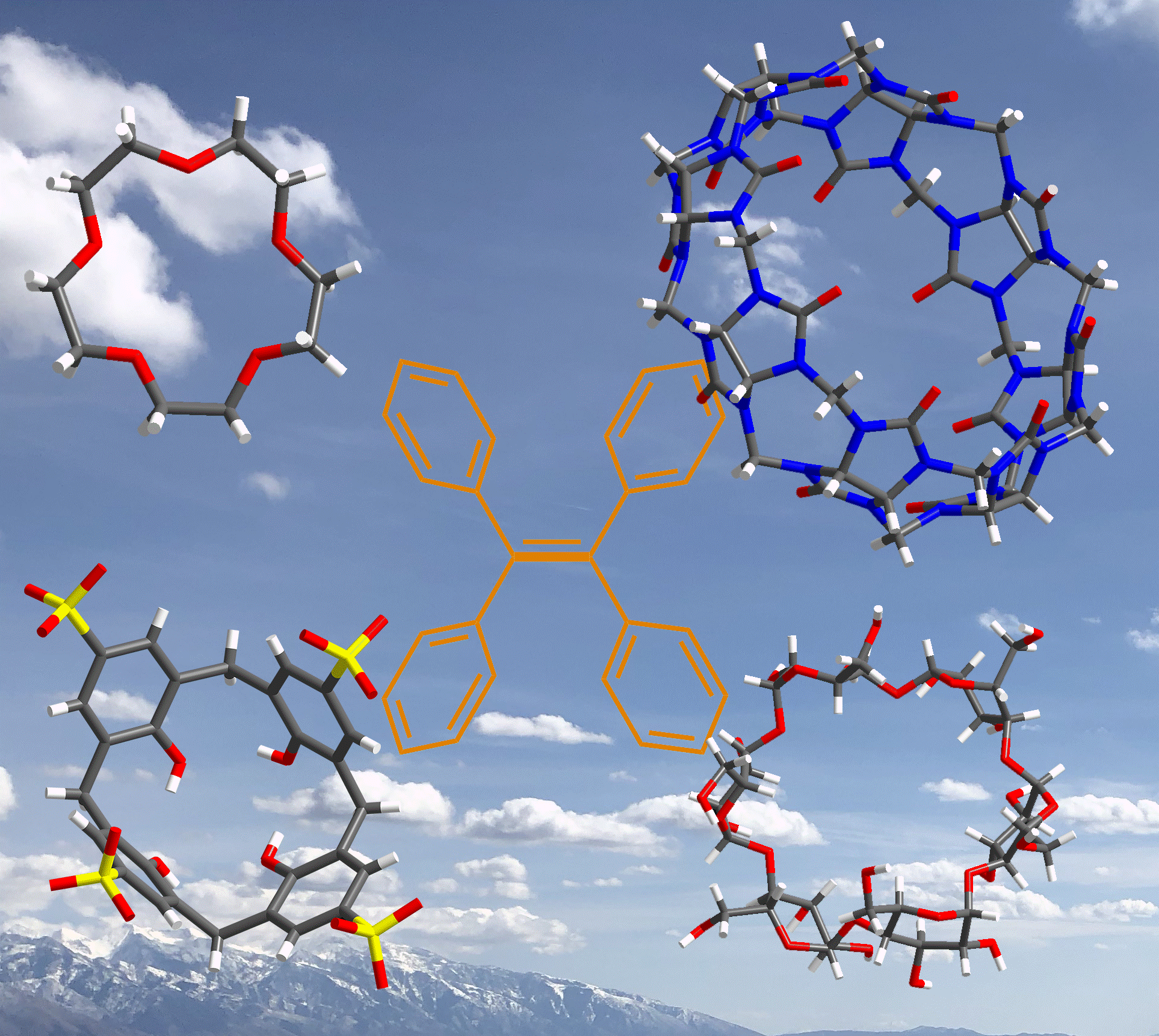
24. 李亚雯; 敖宛彤; 金慧琳; 曹利平* 四苯乙烯衍生物与大环主体在主客体相互作用下的聚集诱导发光, 化学进展, 2019, 31,121-133.
https://manu56.magtech.com.cn/progchem/CN/abstract/abstract12175.shtml
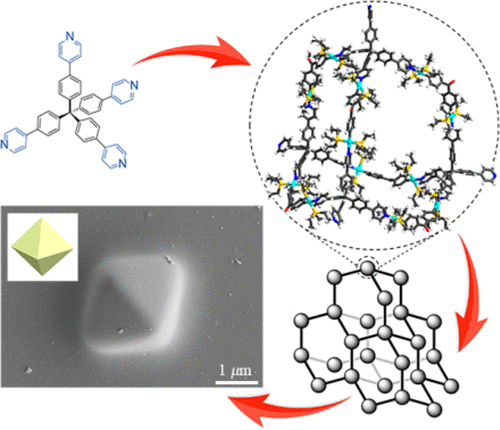
23. Cao, L.*; Wang, P.; Miao, X.; Dong, Y.; Wang, H.; Duan, H.; Yu, Y.; Li, X.; Stang, P. J.* Diamondoid Supramolecular Coordination Frameworks from Discrete Adamantanoid Platinum(II) Cages. J. Am. Chem. Soc. 2018, 140, 7005-7011.
https://pubs.acs.org/doi/10.1021/jacs.8b03856
22. Li, Y.; Dong, Y.; Miao, X.; Ren, Y.; Zhang, B.; Wang, P.; Yu, Y.; Li, B.; Isaacs, L.; Cao, L.* Shape-Controllable and Fluorescent Supramolecular Organic Frameworks through Aqueous Host–Guest Complexation. Angew. Chem., Int. Ed., 2018, 57, 729-733.
https://onlinelibrary.wiley.com/doi/pdf/10.1002/anie.201710553
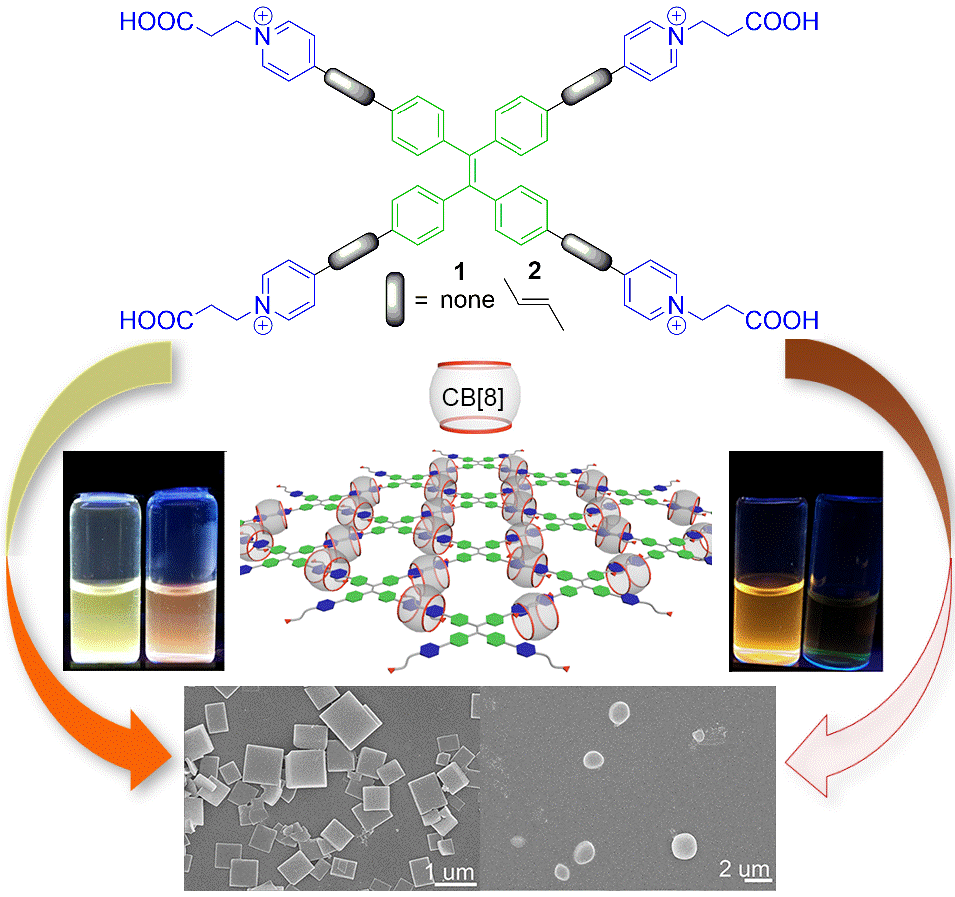
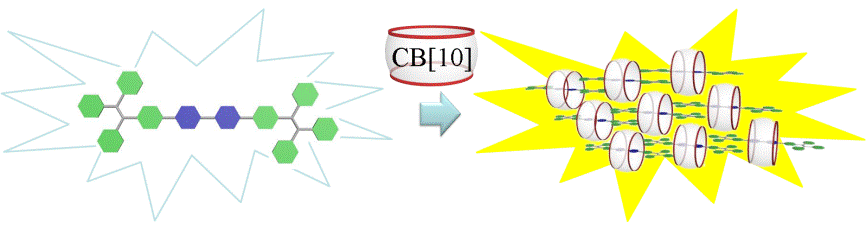
21. Yu, Y.; Li, Y.; Wang, X.; Nian, H.; Wang, L.; Zhao, Y.; Li, J.; Yang, X.; Liu, S.*; Cao, L.* Cucurbit[10]uril-based [2]Rotaxane: Preparation and Supramolecular Assembly-Induced Fluorescence Enhancement. J. Org. Chem. 2017, 82, 5590-5596.
http://pubs.acs.org/doi/pdfplus/10.1021/acs.joc.7b00400
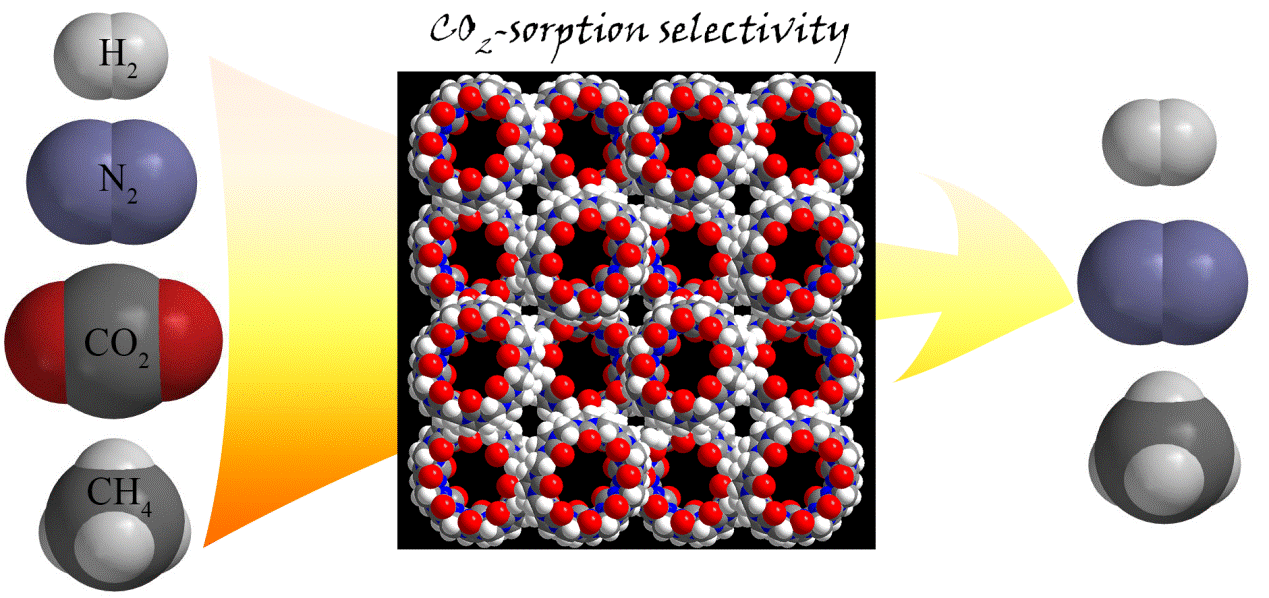
20. Wang, P.; Wu, Y.; Zhao, Y.; Yu, Y.; Zhang, M.*; Cao, L.* Crystalline Nanotubular Framework Constructed by Cucurbit[8]uril for Selective CO2 Adsorption. Chem. Commun. 2017, 53, 5503-5506.
http://pubs.rsc.org/en/content/articlepdf/2017/cc/c7cc02074k
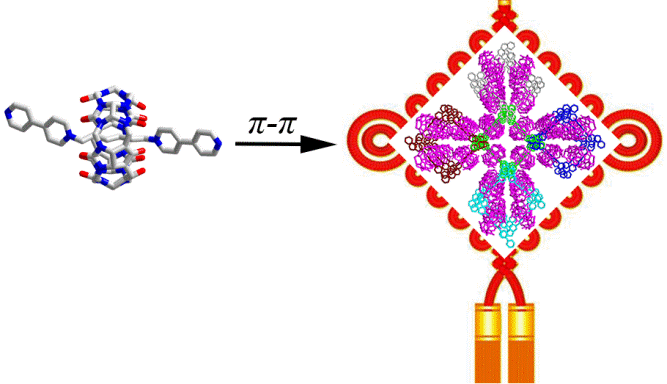
19. Li, J.; Zhao, Y.; Dong, Y.; Yu, Y.; Cao, L.*; Wu, B. Supramolecular Organic Frameworks of Cucurbit[n]uril-Based [2]Pseudorotaxanes in the Crystalline State. CrystEngComm, 2016, 18, 7929-7933.
http://pubs.rsc.org/en/content/articlepdf/2016/ce/c6ce01320a
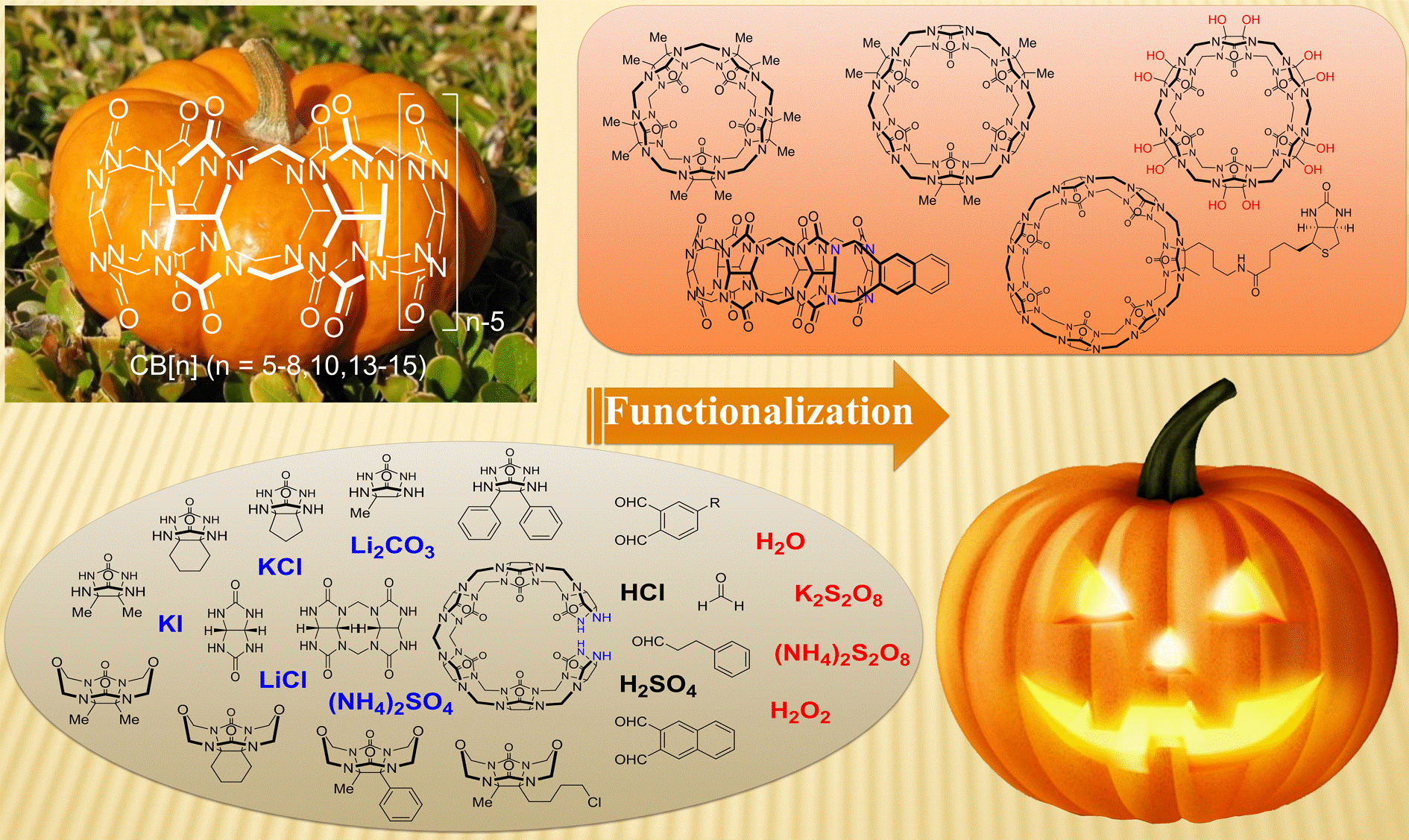
18. Dong, Y.; Cao, L.* Functionalization of Cucurbit[n]uril. Progress in Chemistry,(化学进展) 2016, 28, 1039-1053.
https://manu56.magtech.com.cn/progchem/CN/10.7536/PC160320
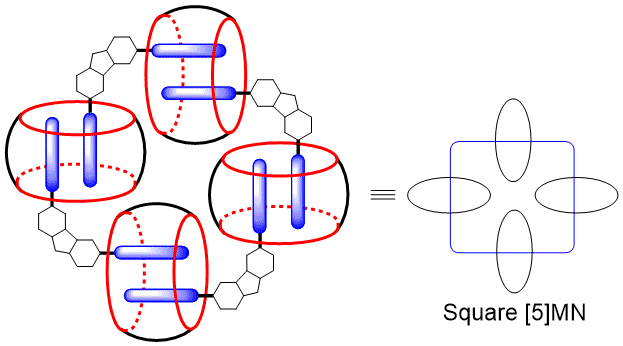
17. Li, J.; Yu, Y.; Luo, L.; Li, Y.; Wang, P.; Cao, L.*; Wu, B. Square [5]Molecular Necklace Formed from Cucurbit[8]uril and Carbazole Derivative. Tetrahedron Lett. 2016, 57, 2306-2310.
https://www.sciencedirect.com/science/article/pii/S0040403916304105?via%3Dihub
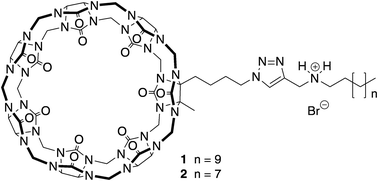
16. Yu. Y.; Li, J.; Zhang, M.; Cao, L.*; Isaacs, L.* Hydrophobic Monofunctionalized Cucurbit[7]Uril Undergoes Self-Inclusion Complexation and Forms Vesicle-Type Assemblies. Chem. Commun. 2015, 51, 3762-3765.
http://pubs.rsc.org/en/content/articlepdf/2015/cc/c5cc00236b
博士后期间工作(2011-2014):
15. Sigwalt, D.; Sekutor, M.; Cao, L.; Zavalij, P. Y.; Hostas, J.; Ajani, H.; Hobza, P.; Mlimaric-Majerski, K.*; Glaser, R.*; Isaacs, L.* Unraveling the Structure-Affinity Relationship between Cucurbit[n]urils (n = 7, 8) and Cationic Diamondoids. J. Am. Chem. Soc. 2017, 139, 3249-3258. (SCI, IF = 13, 一区top)
14. Cao, L.; Skalamera, D.; Zavalij, P. Y.; Hobza, P.*; Mlinarić-Majerski, K.*; Glaser R.*; Isaacs, L.* Influence of Hydrophobic Residues on the Binding of CB[7] toward Diammonium Ions of Common Ammonium•••ammonium Distance. Org. Biomol. Chem. 2015, 13, 6249-6254. (SCI, IF = 3.5)
13. Cao, L.; Šekutor, M.; Zavalij, P. Y.; Mlinarić-Majerski, K.*; Glaser R.*; Isaacs, L.* Cucurbit[7]uril.Guest Pair with an Attomolar Dissociation Constant. Angew. Chem., Int. Ed. 2014, 53, 988-993. (SCI, IF = 13.4, VIP paper and Poster of the frontispiece, 一区top)
12. Zhang, M.#; Cao, L.# (共同第一作者); Isaacs, L.* Cucurbit[6]Uril-Cucurbit[7]Uril Heterodimer Promotes Controlled Self-Assembly Of Supramolecular Networks And Supramolecular Micelles By Self-Sorting Of Amphiphilic Guests. Chem. Commun. 2014, 50, 14756-14759. (SCI, IF = 6.7, 一区)
11. Cao, L.; Isaacs,L.* Absolute and Relative Binding Affinity of Cucurbit[7]uril Towards A Series of Cationic Guests. Supramol. Chem. 2014, 26, 251-258. (SCI, IF = 2.1)
10. Cao, L.; Hettiarachchi, G.; Briken, V.*; Isaacs, L.* Cucurbit[7]uril Containers for Targeted Delivery of Oxaliplatin to Cancer Cells. Angew. Chem., Int. Ed. 2013, 52, 12033-12037. (SCI, IF = 13.4, 一区top)
9. Vinciguerra, B.#; Cao, L.# (共同第一作者); Cannon, J. R.; Zavalij, P. Y.; Fenselau, C.; Isaacs, L.* Synthesis and Self-Assembly Processes of Monofunctionalized Cucurbit[7]uril. J. Am. Chem. Soc. 2012, 134, 13133-12140. (SCI, IF = 11.4, 一区top) (Spotlights on Recent JACS Publications, J. Am. Chem. Soc. 2012, 134, 14265-14266.)
8. Cao, L.; Isaacs, L.* Daisy Chain Assembly Formed from a Cucurbit[6]uril Derivative. Org. Lett. 2012, 14, 3072-3075. (SCI, IF = 6.3, 一区)
7. Lucas, D.; Minami, T.; Iannuzzi, G.; Cao, L.; Wittenberg, J. B.; Anzenbacher, Jr. P.*; Isaacs, L.* Templated Synthesis of Glycoluril Hexamer and Monofunctionalized Cucurbit[6]uril Derivatives. J. Am. Chem. Soc. 2012, 134, 13133-12140. (SCI, IF = 11.4)
博士期间工作(2006-2011):
6. Cao, L.; Wang, J. G.; Ding, J. Y.; Wu, A. X.*; Isaacs,L.* Reassembly Self-Sorting Triggered by Heterodimerization. Chem. Commun. 2011, 47, 8548-8550. (SCI, IF = 6.7, 一区)
5. Cao, L.; Meng, X. G.; Ding, J. Y.; Chen, Y. F.; Gao, M.; Wu, Y. D.; Li, Y. T.; Wu, A. X.*; Isaacs, L.* Nanotubular Non-Covalent Macrocycle Within Non-Covalent Macrocycle Assembly: (MeOH)(12) Encapsulated in Amolecular Clip Cyclododecamer. Chem. Commun. 2010, 46, 4508-4510. (SCI, IF = 6.7)
4. Cao, L.; Ding, J. Y.; Wang, J. G.; Chen, Y.; Gao, M.; Xue, W. J.; Wu, A. X.* Colorimetric Fluoride Sensor Based on a Bisthiourea Functionalized Molecular Clip. Synlett 2010, 2553-2556. (SCI, IF = 2.8)
3. Cao, L.; Ding, J. Y.; Yin, G. D.; Gao, M.; Li, Y. T.; Wu, A. X.* Thioglycoluril as a Novel Organocatalyst: Rapid and Efficient alpha-Monobromination of 1,3-Dicarbonyl Compounds. Synlett. 2009, 1445-1448. (SCI, IF = 2.8)
2. 曹利平; 高蒙; 李义涛; 丁娇阳; 吴彦东; 祝艳平; 佘能芳; 吴安心* 二元组装体集群时的高选择性杂化重组行为. 中国科学B辑: 化学, 2009, 39, 343-349. (国家核心期刊)
1. Cao, L.; Ding, J. Y.; Gao, M.; Wang, Z. H.; Li, J.; Wu, A. X.* Novel and Direct Transformation of Methyl Ketones or Carbinols to Primary Amides by Employing Aqueous Ammonia. Org. Lett. 2009, 11, 3810-3813. (SCI, IF = 6.3) (highlighted by Organic Chemistry Portal: http://www.organic-chemistry.org/abstracts/lit2/649.shtm; ChemInform, 2010, 41, DOI:10.1002/chin.201004082)
参与编写专著:
1. Cao, L.; Zhao, J.; Yang, D.; Yang, X.-J.; Wu, B.* The Chapter 5《Hydrogen Bonding-Driven Anion Recognition》 in Hydrogen Bonded Supramolecular Structures. Springer-Verlag Berlin Herdelberg, 2015, DOI:10.1007/978-3-662-45756-6_5.
https://link.springer.com/chapter/10.1007/978-3-662-45756-6_5

2. Yan, C.; Xiang, S.; Cao, L.* The Chapter《Peptide adaptive detection from chiral AIE》 in 《Encyclopedia of Aggregation-induced Emission》, Springer , 2025.
https://link.springer.com/rwe/10.1007/978-981-97-1574-9_111-1

3. Yan, C.; He, T.; Cheng, L.; Cao, L.* The Chapter 《Biomolecular recognition by water-soluble cationic fluorescent macrocycles》in《Encyclopedia of Aggregation-induced Emission》, Springer , 2025.
https://link.springer.com/rwe/10.1007/978-981-97-1574-9_30-1

*通讯作者(Corresponding author)

